Coronavirus: Made-in-Singapore diagnostics test rolled out at some hospitals here
Sign up now: Get ST's newsletters delivered to your inbox
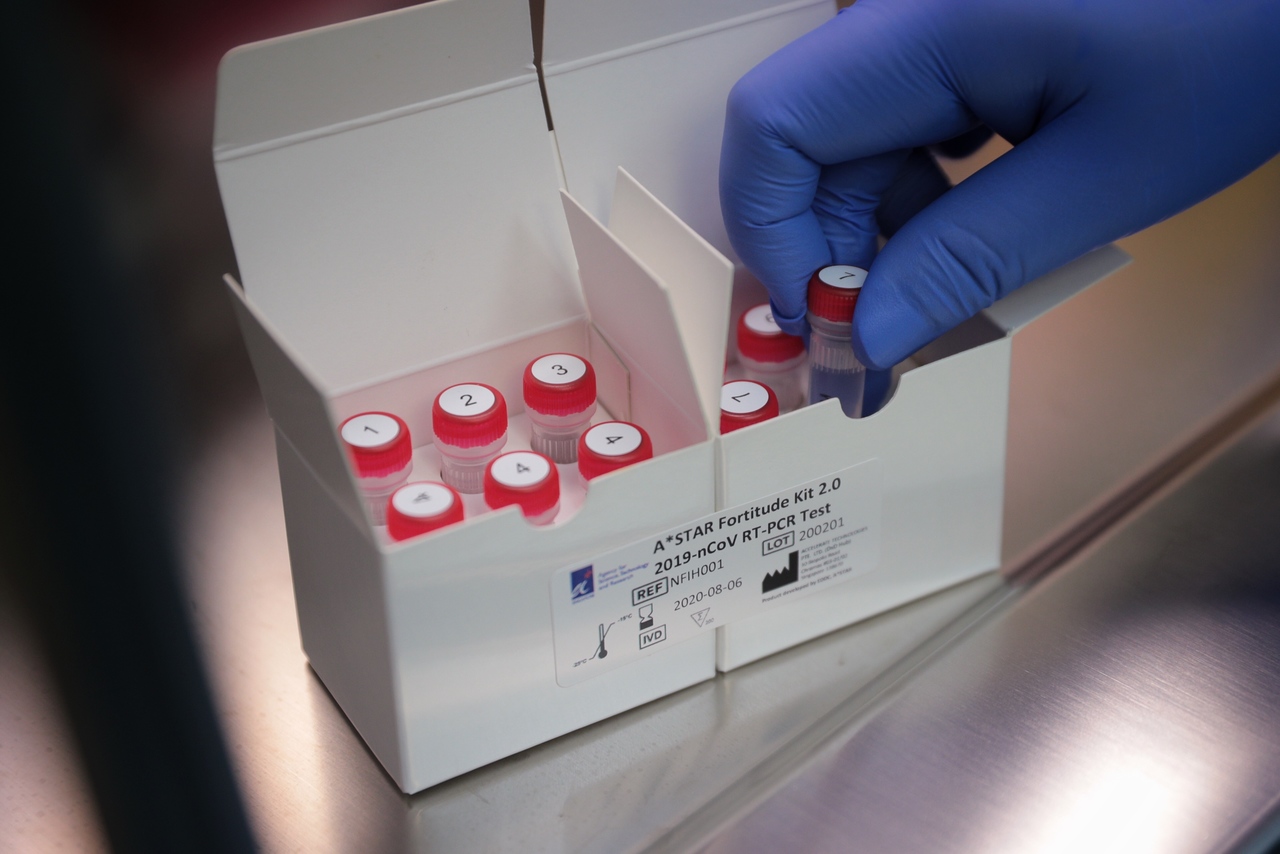
The test kit comprises a pre-packed mix of reagents to test patient samples, which are then fed into a machine that analyses the results.
ST PHOTO: JASON QUAH
SINGAPORE - A made-in-Singapore diagnostic test kit that can detect the presence of the novel coronavirus (2019-nCoV) with high accuracy has been rolled out at some public hospitals here - the first of such diagnostics tests to be implemented locally.
The test kit comprises a pre-packed mix of reagents to test patient samples, which are then fed into a machine that analyses the results. The procedure saves time by allowing other hospitals and laboratories to conduct their own tests.
Plans are in place to scale up their production so such kits can be deployed at other hospitals and laboratories which are not currently offering 2019-nCoV tests, said the Agency for Science, Technology and Research (A*Star), whose scientists developed the pre-packed reagents.
This will widen the network of facilities in Singapore that can accurately screen patients for the coronavirus, reducing the wait time for results and allowing those infected to be treated quickly.
The test kit, which has been approved for use by the Health Sciences Authority, was developed by scientists at A*Star's Experimental Drug Development Centre and Bioinformatics Institute.
The Diagnostics Development (DxD) Hub - a national initiative led by A*ccelerate, the commercial arm of A*Star - supported the verification, validation and production of these tests.
A number of public hospitals here last weekend (Feb 1 to 2) received 5,000 test kits in total.
A*Star declined to name the hospitals that have them but said Singapore had the capability to produce more test kits for other hospitals and laboratories here.
On Saturday, Singapore sent 10,000 of these test kits to China - and another 10,000 more in the coming weeks - to help prevent the further spread of the coronavirus, which has infected more than 31,000 people worldwide in the span of about a month.
The Republic will also be sending three polymerase chain reaction (PCR) machines to China. The PCR machines are an important part of the screening process.
Patient samples are first placed in the tubes that are part of the diagnostics test, before they are fed into the PCR machine for analysis.
As the 2019-nCoV is so new, there has not been a widely implemented test kit for detecting it in Singapore - until now.
It took months before such a test kit was made available during the outbreak of the severe acute respiratory syndrome (Sars) in 2003.
Singapore has been able to produce the test kit for the coronavirus so quickly because of the availability of the genome of the 2019-nCoV, which the scientists in China uploaded early last month, and the advent of PCR technology, which clinical laboratories only started using during the Sars outbreak, said Associate Professor Raymond Lin, director of the National Public Health Laboratory at the National Centre for Infectious Diseases.
The breakthrough comes as Singapore ramps up its disease outbreak response by a notch to orange, after a number of patients were found to be infected with the virus despite having no known links to previous cases or travel history to China.
Under the Disease Outbreak Response System Condition (Dorscon), orange means the outbreak is deemed to have moderate to high public health impact.
The advantage of a diagnostic test kit
Currently, to determine if someone has been infected with 2019-nCoV, samples from the throat, coughed up sputum or lung fluid are first taken from the patient.
These samples are then processed by laboratory technologists at the hospitals.
The technologists first mix reagents together in a tube, before placing the sample in it, and feeding it into a PCR machine.
It can take between two and four hours for the PCR machine to register a reading, but the entire process from sample-taking to getting a diagnosis could take up to a day due to other factors, said Prof Lin.
This includes the time taken to transport the sample to the laboratory, sorting the data, sample processing before the PCR stage, and analysis and reporting.
However, an approved commercially-produced PCR test kit could expedite this process at some laboratories, by reducing the time required for preparing the reagent mix.
Such tests essentially come pre-packed with reagents mixed in the right quantities, and with quality control tubes included. All that is required is for technologists to place the patient sample in the tube before feeding it to the PCR machine.
Explained Prof Lin: "The PCR process is essentially like a recipe that we follow to achieve the outcome, which is a diagnosis. But how successful a recipe is depends on other factors, such as how well the ingredients are mixed and whether the cook can design the right recipe.
"But if the ingredients come pre-packed, like packets of curry powder, for example, then the consistent and quality-tested reagent mix leads to a reliable test result."
Dr Sidney Yee, chief executive officer for A*Star's Diagnostics Development (DxD) Hub, who was part of the team of scientists that helped developed the diagnostics test kit, said its use could cut the time taken in the preparation of reagents by about 30 per cent.
The diagnostics test kit essentially makes the screening procedure easier to administer.
This would allow more laboratories in Singapore - including those with less experience working with reagents required to test for 2019-nCoV - to conduct them as well, without compromising the accuracy of results, said Dr Yee.
The first batch of diagnostics test kit was distributed to the local hospitals as "national service", said Dr Yee, and the next step is to work with local enterprises in the biotech industry to transfer and scale up the manufacturing of the test kit.
Said Dr Yee: "With this diagnostics test (kit), more laboratories in Singapore could be tapped on to screen patients for the coronavirus and shorten the waiting time for the results. The quicker turn-around will also allow infected patients to get the treatment required."


