Singapore could soon make freeze-dried Covid-19 PCR test kit
Sign up now: Get ST's newsletters delivered to your inbox

Director Jimmy Toh showing a sample of freeze-dried polymerase chain reaction assays at the BioAcumen Global lyophilisation centre on March 16, 2021.
ST PHOTO: KUA CHEE SIONG
SINGAPORE - The technique used to preserve food could prove to be a game changer in Singapore's fight against Covid-19 through the development of freeze-dried Covid-19 polymerase chain reaction (PCR) test kits, which would simplify storage and transportation requirements.
Local biotech company BioAcumen Global said the key benefit of lyophilisation, as the technique is called, is that it removes the need for refrigeration currently required for Covid-19 PCR test kits.
Freeze-dried kits can be kept at room temperature without any degradation.
BioAcumen Global officially opened its new lyophilisation facility on Tuesday (March 16).
It has the capacity to freeze-dry 2,400 PCR tests a day and hopes to double this capacity soon.
Currently, the lab freeze-dries PCR kits that detect the African swine fever virus.
BioAcumen Global also produces about 20,000 "wet" Covid-19 PCR test kits a day, which had obtained approval from the Health Sciences Authority in September 2020.
It is currently developing its freeze-dried version of Covid-19 PCR kits. They are expected to be ready in around two months' time.
Covid-19 PCR test kits are currently shipped at low temperatures below 0 deg C. However, with a freeze-dried version of the kit, they can be transported and stored at room temperatures for up to six months.
Lyophilised Covid-19 PCR test kits have been developed elsewhere, such as by United States-based company, Biomeme.
Biomeme's freeze-dried kit was granted emergency-use authorisation by the US Food and Drug Administration last year and has helped to extend shelf life and allow for easier transportation and storage without the need for a cold chain.
The accessibility of such kits has helped many rural communities gain access to Covid-19 PCR testing worldwide. They also facilitate decentralised PCR testing in places such as small clinics, which do not have the infrastructure necessary for conventional PCR tests.
BioAcumen Global on Tuesday also inked a new partnership with biotech company LGC, Biosearch Technologies, which is headquartered in Britain. LGC, Biosearch Technologies manufactures the components in diagnostic kits.
The tie-up will stand to boost the supply chain of lyophilised kits, including those for Covid-19, and accelerate the time they take to reach the market, Mr Jimmy Toh, director of BioAcumen Global said.
LGC, Biosearch Technologies will provide the components for the kits and BioAcumen will assemble before freeze-drying them.
The freeze-dried PCR kit will be slightly more expensive than the wet versions, but it will not be an astronomical figure, Mr Toh added.

BioAcumen Global's first successful freeze-dried PCR kit is being used to detect the African swine fever virus and has been seeing commercial interest from customers around South-east Asia. Almost 200 of these African swine fever virus PCR tests will be shipped to Bangkok next week.
Beyond test kits, freeze-drying could also be used for Covid-19 vaccines.
US-based biotechnology company Arcturus Therapeutics is developing a Covid-19 vaccine with the Duke-NUS Medical School here, and looking at a freeze-dried version of its mRNA vaccine.
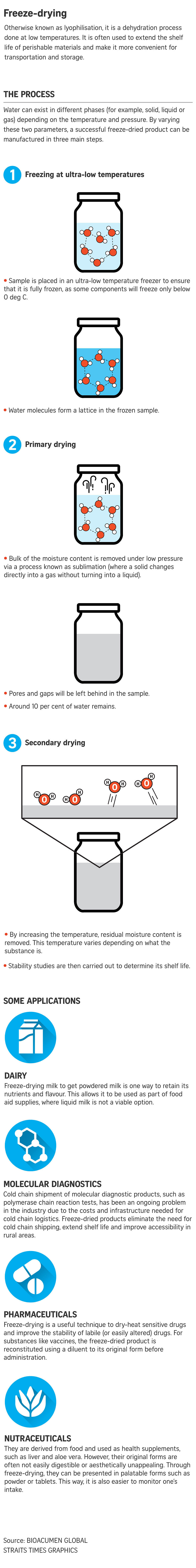
The freeze-drying process is done in three parts. In the case of PCR kits, they are first frozen at ultra-low temperatures of below -80 deg C for four hours to ensure that the contents are completely frozen.
The kits then undergo a process known as primary drying to remove the ice crystals under low pressure. Around 90 per cent of the water molecules are removed at this stage, with the process taking around five hours.
The final stage, known as secondary drying, removes any residual moisture in the sample and needs an additional two hours. The end product has around 1 per cent of water content.
Lyophilising PCR kits is a complex process, and an additional suitable sugar molecule has to be added to the "wet" kits in order to provide the matrix necessary to hold the PCR components together after they have been freeze-dried.
Having the freeze-drying facility housed within an ISO 6 certified cleanroom will also allow in-vitro diagnostic grade PCR kits to be manufactured, Mr Toh said.
Cleanrooms are classified from ISO 1 to 9, according to the cleanliness level of the air inside them, based on the quantity and size of the particles in the air.
BioAcumen Global is also looking to provide lyophilisation services to other companies and research labs to apply freeze-drying into areas such as food science and drugs.