Covid-19 vaccine roll-out gives UK a rare win in battling pandemic
Sign up now: Get ST's newsletters delivered to your inbox
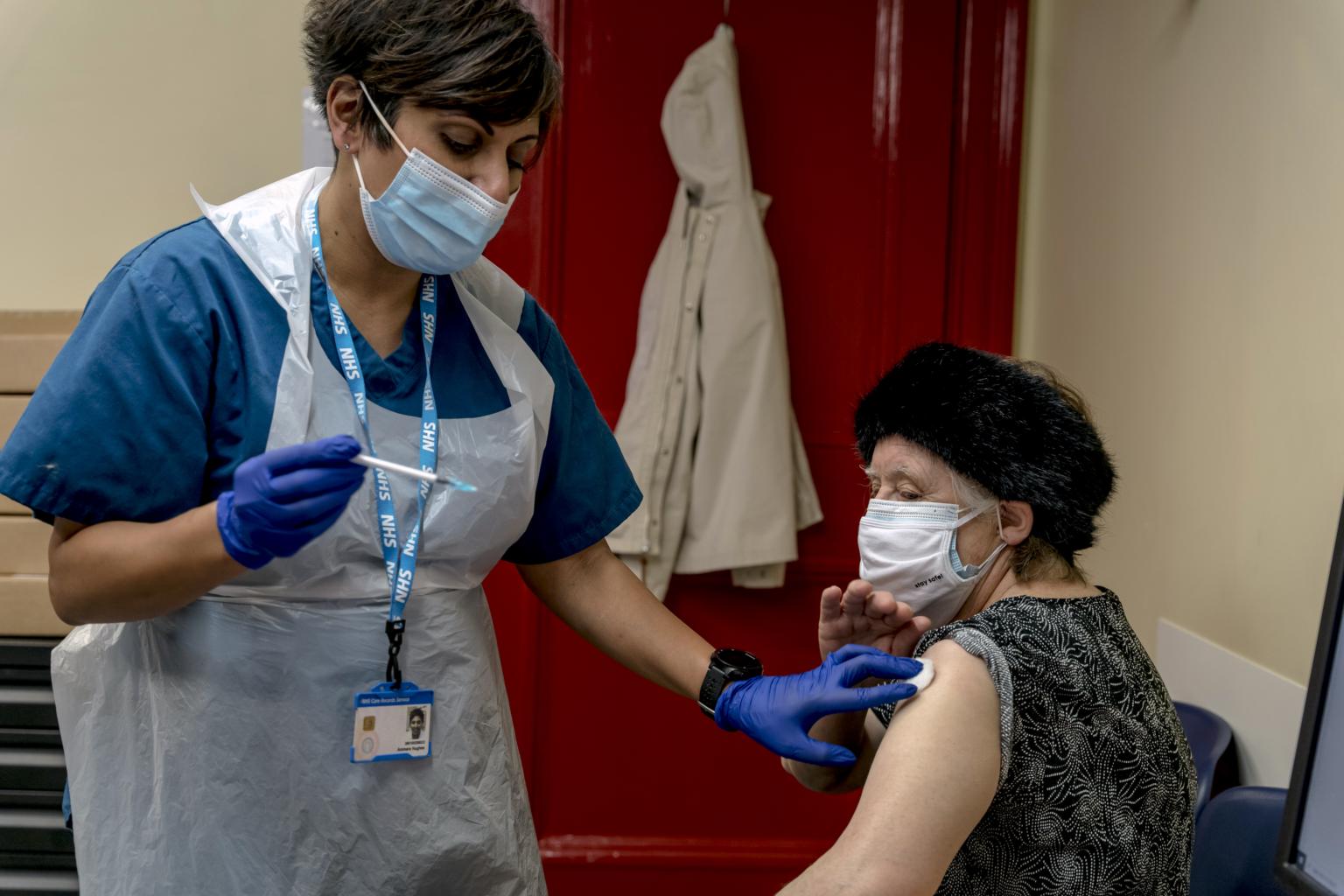
A Pfizer coronavirus vaccine being administered at the Bloomsbury Surgery in London on Jan 28, 2021.
PHOTO: NYTIMES
LONDON (NYTIMES) - When the pizza-sized boxes of the Pfizer vaccine arrived midday on Thursday (Jan 28), an hour behind schedule, it set off a race against the clock at Bloomsbury Surgery, a medical clinic in London's Camden district that has been transformed during the pandemic into a humming vaccination centre.
Because the vaccine could only be refrigerated for three days once it reached the clinic, health care workers knew they had to inject 400 doses a day by Saturday to use up the supply. There was already a line of people waiting for "jabs", so doctors swiftly diluted the vaccine, put the vials on trays and handed them out to a team of assistants.
This is the front line in what has become the most ambitious peacetime mass mobilisation in modern British history. Britain has set up dozens of vaccination centres in sports stadiums, churches, mosques, even an open-air museum in the Midlands, familiar to television views as the set for the popular crime series "Peaky Blinders".
With nearly 8 million people, or 11.7 per cent of the population, having already gotten their first shot, Britain's pace of vaccination is the fastest of any large nation in the world. Only Israel and the United Arab Emirates are moving faster.
The rapid roll-out is a rare success for a country whose response to the coronavirus has otherwise been bungled - plagued by delays, reversals and mixed messages. All of which have contributed to a death toll that recently surged past 100,000 and cemented Britain's status as the worst-hit country in Europe.
Success has brought its own headaches: Doctors now worry about running short of supplies, after a vaccine war erupted between Britain and the European Union. The EU imposed export restrictions on vaccines made in the bloc on Friday after accusing a British-based vaccine maker, AstraZeneca, of favoring its home market.
The divergence between Britain and its European neighbours has prompted some to claim an early windfall from Brexit. Britain's divorce from the European Union helped give it the political leeway to authorise multiple vaccines before the bloc and to swiftly lock up its own production of the vaccine from AstraZeneca and the University of Oxford.
France, by contrast, has vaccinated only 1.8 per cent of its population and Germany 2.6 per cent, according to numbers collected by Our World in Data. That reflects supply shortages that have rippled across the continent, as well as the slow pace of European Union regulators in approving vaccines.
But Britain's success is also a result of back-to-basics decisions by the government of Prime Minister Boris Johnson.
Rather than contracting out the campaign to private companies or building it from scratch, as it did with its costly, ineffective contact tracing operation, the government has put vaccination in the hands of the National Health Service, which despite financial strains is still widely revered by the British public.
Beyond state hospitals, physicians are at the forefront of the programme. Not only does that put trusted local doctors, who are experienced with seasonal flu inoculations, in charge, but it has also allowed these doctors to precisely target the people in the government's highest priority groups.
That is a stark contrast to the more fragmented approach in the United States. While Americans have had to scramble for appointments on finicky online portals and overwhelmed telephone hotlines, British hospitals and physicians have directed the scheduling themselves, allowing them to begin with their oldest and most vulnerable patients.
And while US states use complicated rules to dictate who is eligible for vaccines - which has contributed to slowing the roll-out in some places - Britain has a clear system of prioritising those who, because of their age, are most at risk of dying from the virus, along with the nursing home aides and health care workers who treat them.
While some observers point to Britain's higher tolerance for risk than the European Union, they credit more of the vaccination success to the country's strong scientific base, as well as to "good old-fashioned preparation", said David Goodhart, a writer whose last book, "The Road to Somewhere" explored Brexit-era Britain.
It was not, in any event, typical of Britain's broader response.
Few foreign leaders have struggled with the pandemic like Mr Johnson. He abandoned large-scale contact tracing and resisted imposing a lockdown, then ended up in an intensive care unit himself after contracting the virus.
But during those chaotic early days, his ministers moved to invest in vaccines and secured early contracts with manufacturers. They also recruited Kate Bingham, a British venture capitalist, to lead a government vaccine task force.
In March, the government provided initial funding - £2.6 million (S$4.74 billion) - to the Oxford research team. By May, when the vaccine was still in clinical trials, Britain reached a deal with AstraZeneca to buy tens of millions of doses, three months before the European Union negotiated its purchases.
With worries about vaccine protectionism already flaring, British officials were determined to make any homegrown vaccine quickly and easily accessible to Britons. They spoke to the Oxford team as it negotiated with Merck and other drug companies in search of a partner to mass-produce and distribute the vaccine.
Oxford eventually struck a deal with AstraZeneca, which is headquartered in Cambridge.
"They made it pretty clear to me and others that they wanted to know about the deal, and they were anxious about vaccine nationalism," John Bell, an Oxford professor and member of the government's vaccine task force, said last year, referring to British health officials.
Two plants in England are now manufacturing the vaccine, and a firm in Wales is preparing it for distribution. The British government has said the bulk of its shipments of AstraZeneca vaccines come from that supply chain.
On Friday, EU drug regulators authorised the AstraZeneca vaccine for all adults, sticking to the precedent set last month by Britain's regulator.
Britain, meanwhile, may soon get yet another vaccine.
Novavax, a biotechnology company based in Gaithersburg, Maryland, reported on Friday that its vaccine had been shown to be 89.3% effective in a large-scale trial in Britain. The government has secured 60 million doses, which will be made at a plant in northeast England. If British regulators approve it, the vaccine will be delivered in the second half of 2021.
All told, the British government has spent at least £11.7 billion, in developing, making, buying, and administering vaccines.
"Vaccination is the one thing we've gotten right," said Christina Pagel, a professor of operational research at University College London.
That does not mean the roll-out has been without tensions. With hospitals overrun and a more contagious variant ripping through the country, Britain has bet on giving more people the partial protection of a single dose, rather than quickly giving fewer people the complete protection of two doses.
Doctors whose booster shots have been delayed have been angered by the approach, accusing the government of making them the subjects of a risky new experiment that they worry will render vaccines less effective. Immunologists have raised concerns that a country full of people with only partial immunity could breed vaccine-resistant mutations, while Pfizer said the strategy is not supported by the data gathered in clinical trials.
But the idea of prioritising first shots has gained some traction as countries struggling with the surging virus and shortfalls in vaccine supply look for ways to get partial protection into their population.


