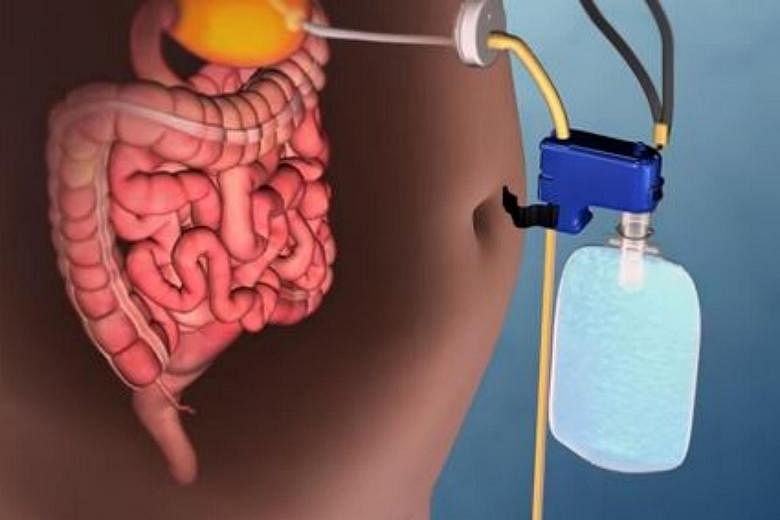
WASHINGTON - A weight loss device that pumps food out of the user's stomach has received a stamp of approval from the United States Food and Drug Administration (FDA).
The AspireAssist is described by the FDA as using a "surgically placed tube" to drain a portion of the stomach's contents after every meal.
In a statement on Tuesday (June 14), the federal agency said the device is intended for patients aged 22 years and older who are obese and have failed to achieve weight loss through non-surgical weight-loss therapy.
Such patients typically have a body mass index of between 35 and 55.
"It should not be used on patients with eating disorders, and it is not intended to use for short durations in those who are moderately overweight," it warned.
Developed by US company Aspire Baratrics, the device has been criticised as "assisted bulimia", reported USA Today. It is currently available in Europe.
Side effects of using the device include stomach pain, irritation, potential leakage and bleeding, among others.
To place the device, surgeons insert a tube in the stomach with an endoscope via a small incision in the abdomen. A "disk-shaped port valve", which lies outside the body, is connected to the tube.
About 20 minutes or 30 minutes after a meal, an external connector is attached to the port valve, which opens it and drains the contents.
The FDA's statement said it takes approximately five to 10 minutes to drain food matter through the tube and into the toilet, with the device estimated to remove about 30 per cent of calories consumed.
From a clinical trial of 111 patients treated with AspireAssist - along with the appropriate lifestyle therapy - it was discovered that they lost an average of 12.1 per cent of their total body weight after one year.
This was compared to the 3.6 per cent weight loss for 60 control patients who only received lifestyle therapy.
Dr William Maisel, the deputy director for science and chief scientist in the FDA's Center for Devices and Radiological Health, said the device would require frequent trips to a doctor to shorten the tube as the patient loses weight.
He added: "Patients need to be regularly monitored by their health care provider and should follow a lifestyle programme to help them develop healthier eating habits and reduce their calorie intake."