US CDC advisers unanimously back Pfizer's Covid-19 vaccine for children ages 5 to 11
Sign up now: Get ST's newsletters delivered to your inbox
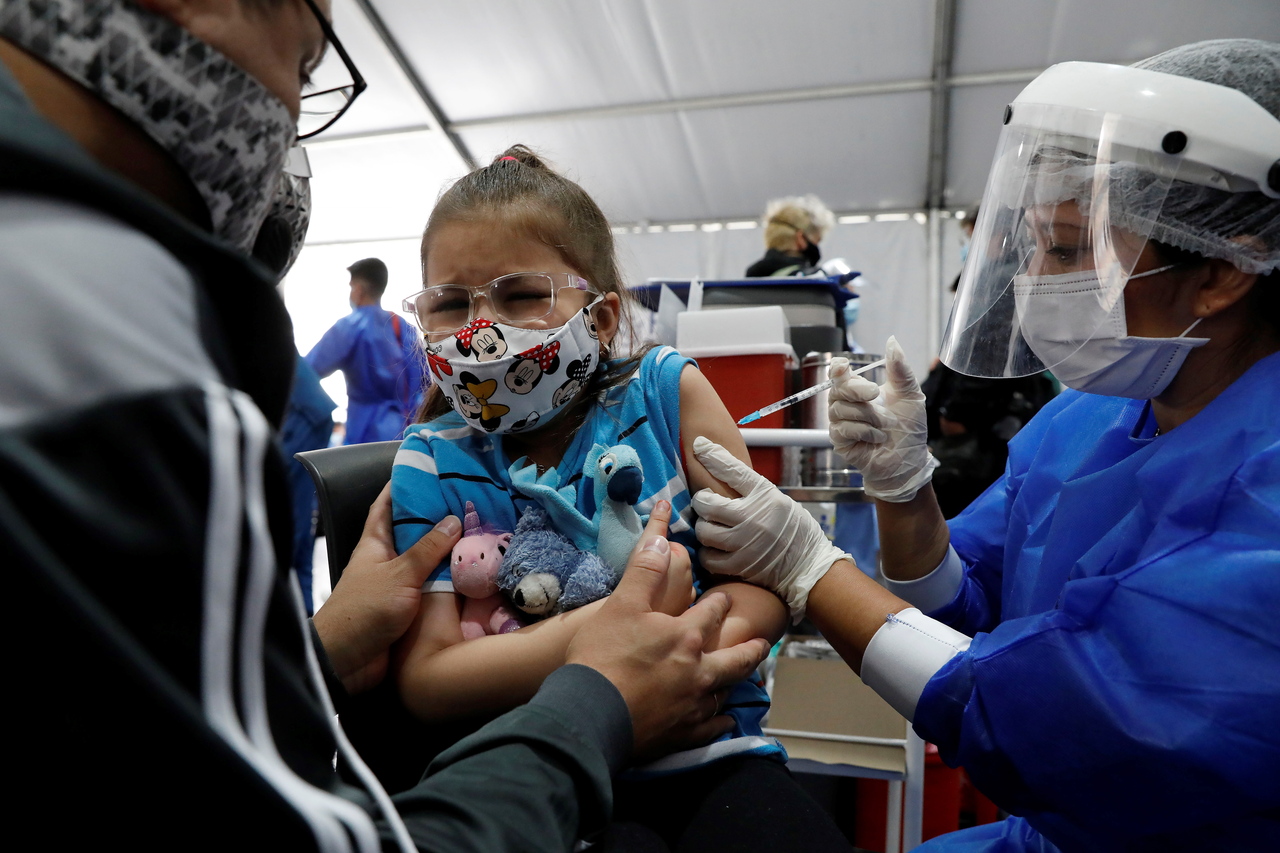
The FDA authorised a 10mcg dose of Pfizer's vaccine in young children.
PHOTO: EPA-EFE
WASHINGTON (BLOOMBERG, REUTERS) - Younger children across the US are now eligible to receive Pfizer's Covid-19 vaccine, after the head of the Centres for Disease Control and Prevention (CDC) granted the final clearance needed for shots to begin.
CDC director Rochelle Walensky on Tuesday (Nov 2) recommended the vaccine for children from five to 11 years old.
The decision ushers in a new phase in the US pandemic response, widening access to vaccines to some 28 million more people at the same time that Americans who received shots earlier in the pandemic are lining up for booster doses.
The CDC's Advisory Committee on Immunisation Practices (ACIP), a group of external experts, earlier voted 14-0 in favour of giving children the shot, developed by Pfizer and BioNTech, after it was cleared last Friday by US regulators.
Administered in two injections three weeks apart, the vaccine is one-third the dose authorised for adults.
"Today is a monumental day in the course of this pandemic, and one that many of us have been very eager to see," Dr Walensky said at the opening of the panel's all-day meeting.
"For almost two full years, schools have been fundamentally changed; there have been children in second grade who have never experienced a normal school year."
A surge in infections fuelled by the Delta variant and the return of in-person learning have increased calls for younger children to be immunised.
Although children generally do not get as sick as adults from Covid-19, many parents and caregivers are keen to give children - as well as older and ill people who interact with them - a shield against infection.
The safety data in children looked very good, said Dr Camille Kotton, an ACIP panel member, adding that she would feel comfortable having her own children immunised if they were in that age group.
Pfizer and BioNTech said their vaccine showed 90.7 per cent efficacy against the coronavirus in a clinical trial of children aged five to 11.
"We have accumulated a tremendous amount of safety data with hundreds of millions of Americans," said Dr Kotton, who is also the clinical director of transplant and immunocompromised host infectious diseases at Massachusetts General Hospital in Boston, in an interview after the vote.
Children should be vaccinated "both to prevent death as well as to prevent major long-term effects of having this devastating infection", she said.
Myocarditis risk
While CDC advisers recommended approval of the shot, some expressed concern about myocarditis, an inflammatory heart condition that has been seen in some recipients.
Health officials have been paying close attention to the risks posed by myocarditis following the shot compared with the overall risks of Covid-19.
Dr Matthew Oster, a paediatric cardiologist at Children's Healthcare of Atlanta, said he believes five- to 11-year-olds have a relatively low risk of developing myocarditis from the shots.
Dr Oster, who is also on the CDC Covid-19 Response vaccine task force, said he does not think the risk "is nearly to the extent" of cases seen in older adolescents and young adults.
"We will watch and see for sure," he added.
Dr Oster said he made that determination based on the general epidemiology of myocarditis, since the paediatric trials were too small to detect such an effect.
The US Food and Drug Administration (FDA) has authorised a 10mcg dose of Pfizer's vaccine in young children. The original shot given to those aged 12 and older is 30mcg.
Pfizer is working with regulators on the handling and distribution of its children's vaccine to accommodate paediatricians administering it. The paediatric vaccine can be sent in packs of just 10 vials, compared with the 195-vial packs of standard vaccines.
Children's vaccine vials contain 10 doses after dilution, while adults carry just six.
Storage is also easier for the children's vaccine, which can be kept in a refrigerator for 10 weeks, or six weeks longer than shots for adolescents and adults.
To differentiate the doses from the purple-capped adult vial, paediatric vials have orange caps.
Roll-out preparations
Officials have been preparing for the roll-out for weeks, and 15 million doses of the Pfizer vaccine specifically formulated for younger children began shipping within minutes of authorisation, Mr Jeff Zients, President Joe Biden's coronavirus response coordinator, said on Monday.
Medical professionals at schools, paediatricians' offices and pharmacies around the country may begin giving them as early as this week, he said in a press call. The programme will be fully up and running the week of Nov 8, Mr Zients said.
Moderna said on Sunday that the FDA was delaying a decision on authorising its Covid-19 vaccine for children aged 12 to 17, citing concerns about myocarditis. The FDA review may not be completed before January, the company said in a statement on Sunday.
The delay will not affect the vaccine roll-out for teenagers, because Pfizer's vaccine is available for the age group, Dr Walensky said at the Monday briefing.
"The thing that's most important is we have a vaccine for our adolescents that is the age demographic under consideration for Moderna," she said.
The US government and Pfizer have already begun distributing the vaccine in preparation for a widespread roll-out for children, many of whom are back in school for in-person learning.
"We've shipped to dozens of states already over the weekend and Monday," Pfizer chief executive Albert Bourla said in an interview. "There is a Herculean effort so there will be doses available everywhere."
Earlier this week, the White House said the United States has enough supply of the Pfizer-BioNTech vaccine for all 28 million children aged five to 11.
While some children may be able to get their first shots as soon as Wednesday, the plan is for the US paediatric vaccine programme to be running at full strength by next week, a Biden administration official said.
Only a few other countries, including China, Cuba and the United Arab Emirates, have so far cleared Covid-19 vaccines for children in this age group and younger.
In the US, around 58 per cent of the population is fully vaccinated, lagging behind other nations such as Britain and France.
The share of young children who receive the shots may be even lower. Only about 47 per cent of US youth aged 12 to 15 are vaccinated.
American states with the highest adult Covid-19 vaccination rates are planning a big vaccine push compared with states where hesitancy remains strong, potentially widening the gaps in protection nationwide, public health officials and experts have said.


