Treating Alzheimer’s very early offers better hope of slowing decline, study finds
Sign up now: Get ST's newsletters delivered to your inbox
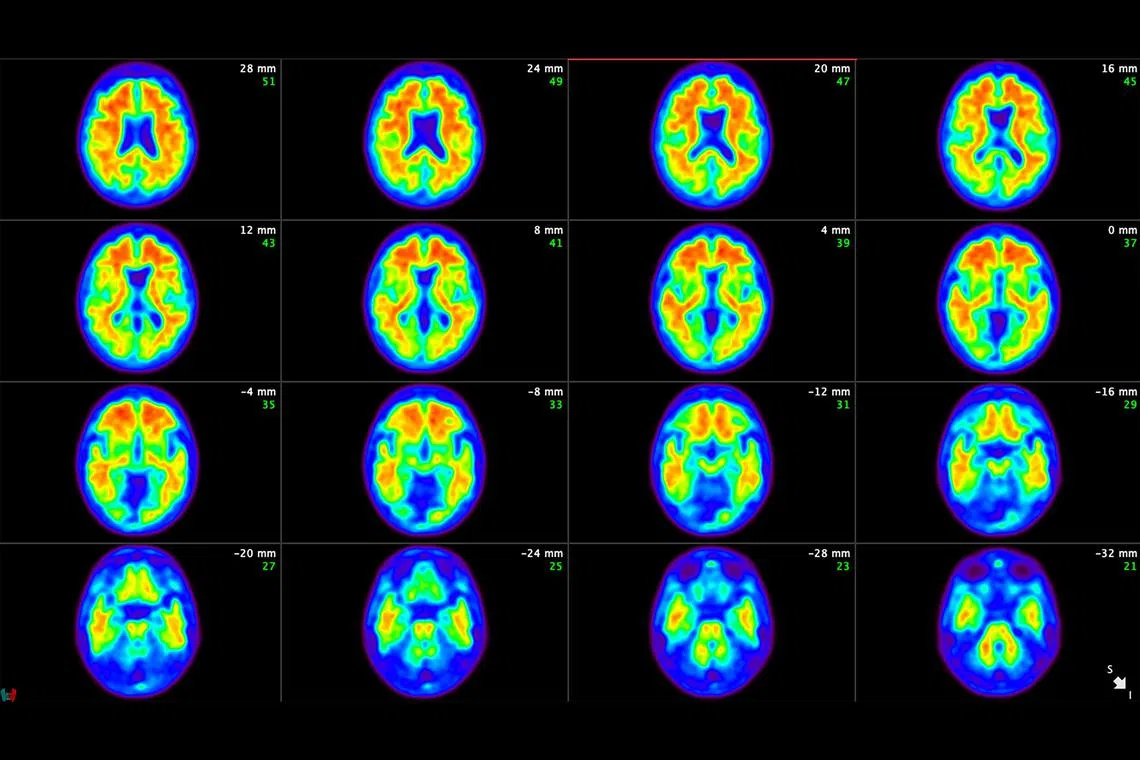
Brain scans from a patient in the clinical trials of donanemab with amyloid plaque being removed from the patient's brain.
PHOTO: NYTIMES
Follow topic:
NEW YORK - Treating Alzheimer’s patients as early as possible – when symptoms and brain pathology are mildest – provides a better chance of slowing cognitive decline, a large study of an experimental Alzheimer’s drug presented on Monday suggests.
The study of 1,736 patients reported that the drug, donanemab, made by Eli Lilly,
For people at that earlier stage, donanemab appeared to slow decline in memory and thinking by about 4½ to 7½ months over an 18-month period compared with those taking a placebo, according to the study, published in the journal Jama.
Among people with less of the protein, called tau, slowing was most pronounced in those younger than 75 and those who did not yet have Alzheimer’s but had a pre-Alzheimer’s condition called mild cognitive impairment, according to data presented on Monday at the Alzheimer’s Association International Conference in Amsterdam.
“The earlier you can get in there, the more you can impact it before they’ve already declined, and they’re on this fast slope,” Dr Daniel Skovronsky, Eli Lilly’s chief medical and scientific officer, said in an interview.
“No matter how you cut the data – earlier, younger, milder, less pathology – every time, it just looks like early diagnosis and early intervention are the key to managing this disease,” he added.
The findings and the recent approval of another drug that modestly slows decline in the early stages of Alzheimer’s, Leqembi, signal a potentially promising turn in the long, rocky path toward finding effective medications for Alzheimer’s, a brutal disease that plagues more than six million Americans.
Donanemab is currently being considered for approval by the US Food and Drug Administration (FDA).
Donanemab and Leqembi (also known by the scientific name lecanemab) have not been compared directly with each other in research studies.
The individual trials of the two drugs differ in design and other aspects, making it hard to say which medication might be more effective.
Each drug poses significant safety risks, especially swelling and bleeding in the brain, which, while often mild, can be serious in some cases.
The donanemab trial had higher rates of swelling and bleeding than the Leqembi trial, but comparisons are difficult because of differences in patients and other factors.
Neither drug reverses or repairs brain damage caused by the disease. Many Alzheimer’s experts therefore consider them to be only a first step in a potentially fruitful direction.
“Whether the harms of these drugs are balanced by their modest clinical benefits will ultimately require more data,” three geriatricians wrote in an editorial published Monday in Jama.
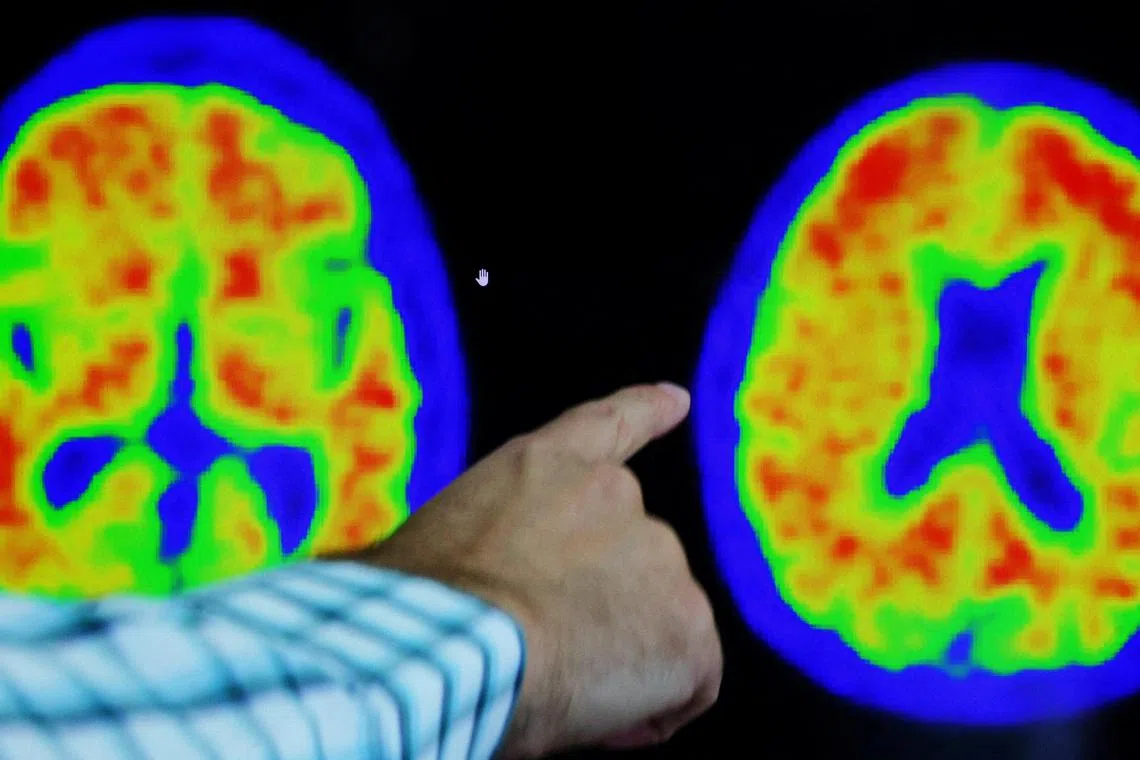
The study of 1,736 patients reported that the drug, donanemab, can modestly slow the progression of memory and thinking problems in early stages of Alzheimer’s.
PHOTO: REUTERS
Three deaths were linked to donanemab in its clinical trial, the study reported.
Three participants in trials of Leqembi also died, after experiencing brain swelling and bleeding.
But Eisai, the Japanese company that makes Leqembi along with the company Biogen, based in Boston, Massachusetts, has said it is unclear if the drug contributed to those deaths because those patients had complex medical issues.
The two drugs attack another protein, called amyloid, which clumps into plaques in the brains of Alzheimer’s patients.
Over years of study, other anti-amyloid drugs failed to show that targeting amyloid could slow memory or thinking problems.
And the FDA’s decision in 2021 to give a type of conditional approval to the anti-amyloid drug Aduhelm while acknowledging uncertainty about whether it was beneficial generated controversy, congressional investigations and reluctance to prescribe it.
Donanemab and Leqembi, infusions that are administered intravenously, are the first amyloid-attacking drugs with clear evidence of slowing cognitive decline early in the disease.
But some Alzheimer’s experts say the slowing is so modest it is unclear if it will be noticeable to patients and families.
Leqembi patients, who received infusions every two weeks for 18 months, declined 27 per cent more slowly than patients receiving a placebo – a difference of less than half a point on an 18-point cognitive scale that assesses functions such as memory and problem-solving.
Donanemab and Leqembi, infusions that are administered intravenously, are the first amyloid-attacking drugs with clear evidence of slowing cognitive decline early in the disease.
PHOTO: REUTERS
On the same scale in the donanemab trial, the overall group of patients receiving the drug, delivered in monthly infusions, declined 29 per cent more slowly than the placebo group – or a difference of seven-tenths of a point.
Some Alzheimer’s experts say that for slowing of decline to be clinically meaningful or noticeable, the difference between a drug and a placebo must be at least one point.
Other aspects of the donanemab trial are likely to be especially intriguing to Alzheimer’s experts.
Patients stopped receiving donanemab and were switched to a placebo if their amyloid was cleared below a certain threshold.
About half reached the threshold within a year, and their decline kept slowing even after they stopped receiving donanemab.
Lilly scientists have estimated that it would take nearly four years for amyloid levels to bump up over the threshold again. It is uncertain whether slowing of decline would continue as amyloid begins accumulating again.
The difficulty of predicting if these drugs will be meaningful in daily life is reflected in the experience of a patient in another donanemab trial.
About four years ago, 67-year-old Jim Sirois of Berlin, Connecticut, began having trouble finding words during conversations and would forget which items to buy at the grocery store.
In November 2021, Mr Sirois, a former power company electrician, started receiving monthly donanemab infusions in a trial comparing whether the drug clears more amyloid than the drug Aduhelm does.
His wife Sue, a former middle school maths teacher, said in an interview arranged by Eli Lilly that donanemab cleared the plaques and that treatment was stopped after about 13 months.
But the couple said they do not know if the medicine slowed Mr Sirois’ cognitive decline.
While her husband’s symptoms have not worsened significantly, Mrs Sirois said: “There were some things he could do without problems last summer that he has difficulty doing this summer.”
Mr Sirois is now unable to hook up their pool vacuum or put string into their grass trimmer.
“He just has a lot of difficulty with planning and anything that has multi steps,” she said.
Even bowling, an activity he excels at, has been affected.
His aim can be less targeted now and, although he recently bowled a perfect game, “his average is probably a good 20 pins lower than it used to be”, she said.
“I don’t know if the drug has helped him or not,” Mrs Sirois said. “I can’t tell.”
But, she added: “Whatever we can do to slow the progression or at least have some hope of slowing the progression is what I would want to do.” NYTIMES

