Clinical trials of coronavirus drugs are taking longer than expected
Sign up now: Get ST's newsletters delivered to your inbox
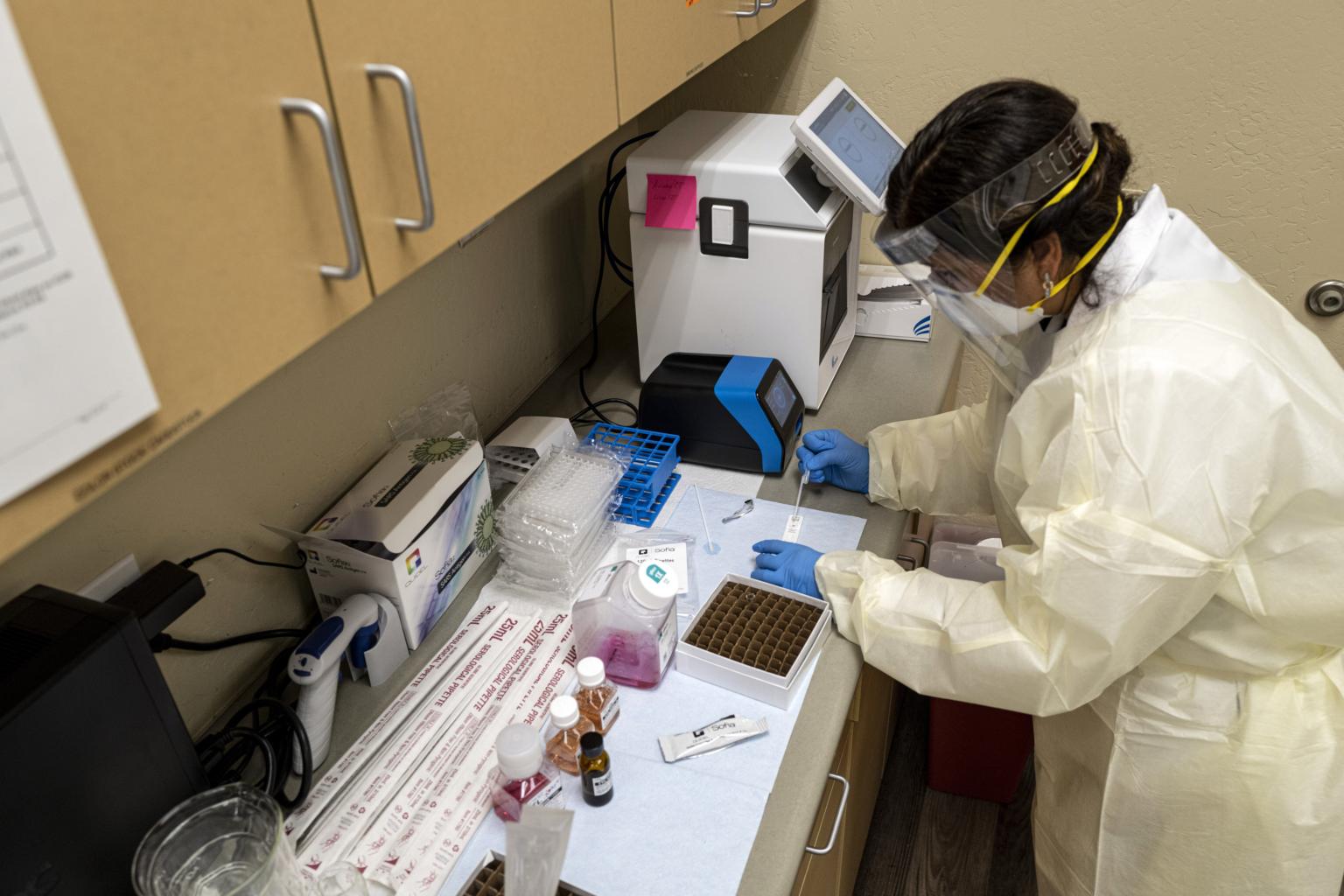
Testing delays, difficulty finding trial sites and challenges to enrolling patients have hindered the research.
PHOTO: NYTIMES
Follow topic:
NEW YORK (NYTIMES) - As the coronavirus pandemic continues to wreak havoc in the United States and treatments are needed more than ever, clinical trials for some of the most promising experimental drugs are taking longer than expected.
Researchers at a dozen clinical trial sites said that testing delays, staffing shortages, space constraints and reluctant patients were complicating their efforts to test monoclonal antibodies, man-made drugs that mimic the molecular soldiers made by the human immune system.
As a result, once-ambitious deadlines are slipping. The drugmaker Regeneron, which previously said it could have emergency doses of its antibody cocktail ready by the end of summer, has shifted to talking about how "initial data" could be available by the end of September.
And Eli Lilly's chief scientific officer said in June that its antibody treatment might be ready in September, but in an interview this week, he said he now hopes for something before the end of the year.
"Of course, I wish we could go faster; there's no question about that," said the Eli Lilly executive, Dr Daniel Skovronsky. "I guess in my hopes and dreams, we enroll the patients in a week or two, but it's taking longer than that."
A spokesman for Regeneron, Ms Hala Mirza, said that all clinical trials involved an early learning period and that the company was "seeing positive momentum in recent days" as it sent testing machines to some research sites and broadened criteria to allow more patients to participate.
While much of the world's focus has been on the race to create a coronavirus vaccine, new drugs could also help curb the pandemic by making the disease less deadly. Because drugs are typically tested in sick patients in smaller clinical trials, they can also be developed more quickly than vaccines.
Eli Lilly and Regeneron are pursuing two of the most closely watched treatments: lab-engineered antibodies that could either fight off the virus in patients who are already sick or prevent infections in those who have been exposed.
Although the Trump administration has heavily favoured investment in vaccines, Regeneron has won deals from the federal government worth more than US$500 million (S$685 million) to ramp up manufacturing of its antibody treatment.
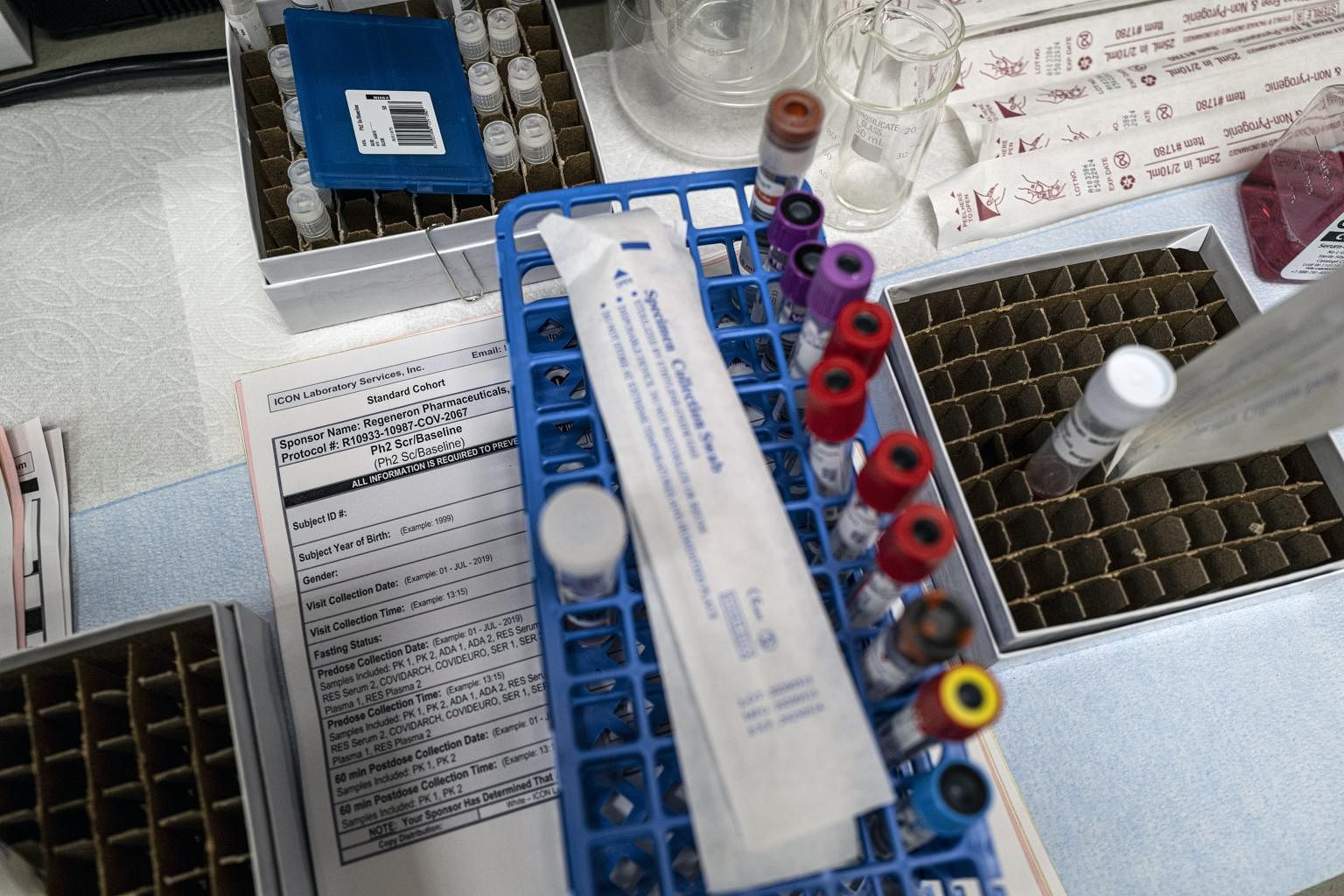
Both companies rushed to develop their products in record time and began large studies this summer at dozens of hospitals and clinics around the country.
They are testing various groups of patients, such as those who are positive but not yet sick enough to be hospitalised and those who have been exposed to the virus from someone already infected. All of the trials compare the experimental drugs to a placebo, or sham treatment.
The fast-moving disease has presented opportunities and challenges for the researchers testing antibodies. As the number of infections mounted in states like Florida, Texas and Arizona, there was no shortage of patients who would be eligible for trials. But at the same time, the outbreaks overwhelmed the very hospitals that would be overseeing the studies.
In remote meetings with doctors at trial sites around the country, Dr Skovronsky said some had to step out to care for patients who required emergency intervention.
"That doesn't happen when you're setting up diabetes trials or cancer trials," he said. "We've had investigators say, 'Look, I'd love to do research, but I don't have time to set up a new trial. I've got an ICU full of patients.'"
One major hurdle has been testing. In both of the outpatient trials run by Eli Lilly and Regeneron, doctors must compete with a ticking clock. According to the rules of the Regeneron trial, a patient must be treated with the antibodies within seven days of the onset of symptoms. Both the Regeneron and Eli Lilly trials require giving the drug within three days of taking a positive test.
But with turnaround times in some areas lagging for five days or more, keeping within those time frames has proved difficult.
Dr Anita Kohli, director of research at Arizona Clinical Trials, a Regeneron trial site in Mesa, Arizona, said she got a rush of people wanting to volunteer for the outpatient study earlier this summer, when the outbreak was peaking in her state.
"Our phones were ringing probably off the hook the first two weeks," she said.

But as labs were inundated with samples, straining supply chains and delaying results, Dr Kohli said enrolling patients became difficult.
"If people are getting tested at these facilities, but they don't have the results, then how do we enroll them in trials?" she said.
Other researchers said finding the right spot to give the experimental treatment to outpatients was complicated. Patients typically get tested at a drive-through site, and then return home, rarely visiting a hospital or clinic unless their condition gets worse. Bringing them into a medical centre for treatment further strains the system and risks infecting patients and healthcare workers who don't have Covid-19.
Some sites have resisted bringing a highly infectious person onto their medical campuses, potentially sharing a lobby or lift with people who do not have the virus. Once there, they must spend a few hours receiving an infusion of the treatment as well as getting blood tests and completing paperwork required for the study.

At some hospitals, officials have been able to use existing facilities. In Tyler, Texas, the UT Health North Campus medical centre is an old tuberculosis hospital, with rooms that use negative air pressure to prevent viruses from spreading.
But in other locations, like Holy Cross Hospital in Fort Lauderdale, Florida, finding the right spot has been a struggle. Dr Joshua Purow, who is overseeing the Eli Lilly outpatient trial at the hospital, rushed to get his site ready once he saw that infections were rising in the area.
But Holy Cross turned down his first choice, a corner of the emergency department, out of fears that the space would be needed for more severe Covid-19 patients. The idea of installing an outdoor tent was deemed too complicated, and refurbishing a room in a nearby office building would take precious weeks.
Weeks passed before Dr Purow finally secured a place to run the trial. It was in the emergency department, the first place he had requested.
"We finally have it all set up to go," Dr Purow said. "But now our numbers are declining a little bit. We're not seeing as much as we thought we would."
So far, he said, he has enrolled just one participant out of a hoped-for 25. Overall, the Eli Lilly outpatient trial is aiming for including 400 patients. The similar Regeneron study has a goal of enrolling about 1,500 patients.
Not every trial site is seeing such hurdles. Dr Jason Morris, who is overseeing the Eli Lilly study at his physician practice, Imperial Health, in Lake Charles, Louisiana, has already exceeded his goals and has enrolled about 45 patients. Dr Morris said he or another doctor calls each person who tests positive for the virus at the group's urgent care clinic and tells them about the study.
He said he explains that the study is examining whether Eli Lilly's antibody can stop the virus from invading cells and replicating.
"When I break it down pretty simply like that, people are like, 'Oh, let's do that,'" he said.
But some patients are reluctant to participate. Many people associate clinical trials with treatments that are given in life-or-death situations and don't want to risk taking an experimental drug for an illness they may overcome on their own.
Others have the opposite rationale: They don't want to go through the hassle of a trial only to receive a placebo.

