All that helps a human live, breathe and function develops from a single embryo.
Researchers have now found a way to better scrutinise the early days of a budding human without having to obtain blastocysts - or early-stage embryos - from in-vitro fertilisation (IVF) procedures.
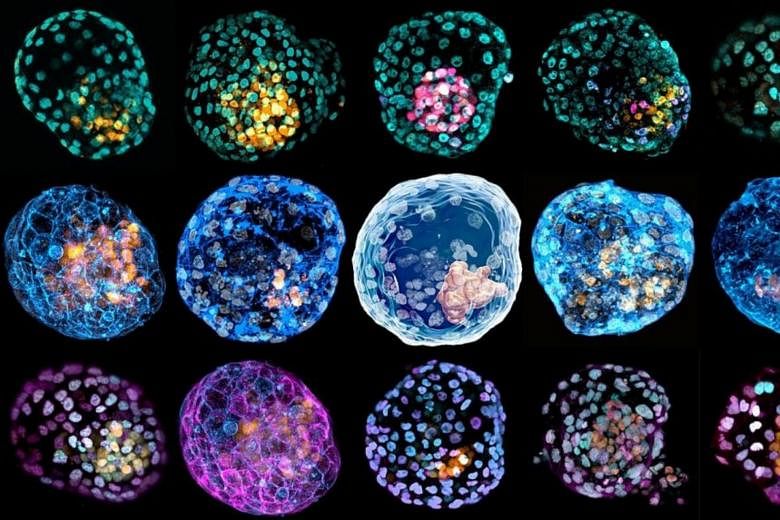
An international scientific team has developed a model blastocyst by "reprogramming" skin cells.
This iBlastoid, as the model is called, can be used to learn more about early miscarriage and other issues without the use of human blastocysts, say the researchers.
But they stress that the technique does not generate embryos - only models that have the same three-dimensional structure and architecture of a blastocyst. For instance, the iBlastoid has an internal cavity and possesses structures made of cells similar to the ones found inside a blastocyst.
"An iBlastoid is not generated using an egg or sperm and has limited ability to develop beyond the first few days," said Assistant Professor Owen Rackham from the Duke-NUS Medical School in Singapore, one of the research members involved in the effort.
"Based on pre-clinical work in mice, these iBlastoids are not viable as embryos."
The means that the use of iBlastoids can alleviate many of the ethical considerations associated with donated IVF blastocysts, easing the controversy of performing research in this area, added Prof Rackham, who is from the Duke-NUS' Cardiovascular and Metabolic Disorders Programme.
The findings of the research team, led by scientists from Monash University in Australia, were published last Wednesday (March 17) in the scientific journal Nature.
Monash's Professor Jose Polo said: "iBlastoids will allow scientists to study the very early steps in human development and some of the causes of infertility, congenital diseases and the impact of toxins and viruses on early embryos without the use of human blastocysts and importantly, at an unprecedented scale, accelerating our understanding and the development of new therapies."
All the cells in a human body have the same genetic material, called DNA, which is like an instruction manual for the body. But different cells have different functions - for example, cells found in the heart and those in the brain serve different purposes - and so they take instructions from different parts of the DNA manual.
A specific class of genes, called transcription factors, play the role of the overseer, coordinating the cues that the various cell types take from the DNA.
But these overseers can be "swayed": Earlier research had found that cell types can be artificially controlled by forcibly "turning on" transcription factors in a cell type they typically would not be activated in.
Prof Rackham said there were a number of challenges to engineering cell conversions, such as having the knowledge of the correct set of transcription factors required to create each cell type, as well as of the conditions under which reprogrammed cells can thrive.
"These problems are hugely complex and are very difficult to solve using traditional experimental techniques," he added.
But the researchers prevailed after building on earlier scientific work.
Prof Rackham said his research group at Duke-NUS looks at combining machine-learning and single-cell sequencing to predict how to make any human cell type.
An earlier collaboration between his team and the Monash researchers had identified the transcription factors required to programme skin cells into induced trophoblast stem cells - cells that are similar to those from human blastocysts that give rise to the placenta.
That research, as well as work done by other groups using a similar process to create embryonic stem cell-like cells, paved the way for the development of the iBlastoid.
After reprogramming the skin cells towards induced trophoblast and embryonic stem cells, the reprogrammed cells were placed into a three-dimensional "jelly" scaffold, where they organised into blastocyst-like structures - or iBlastoids.
Prof Rackham said the discovery demonstrates the plasticity of human cells and the enormous potential that can be unlocked by understanding how to engineer cell fate systematically.
He added: "I believe that the discovery of iBlastoids will democratise research in early development and infertility, making it more accessible to a larger range of research groups and ultimately accelerate the discovery of treatments in these otherwise understudied but hugely important areas."