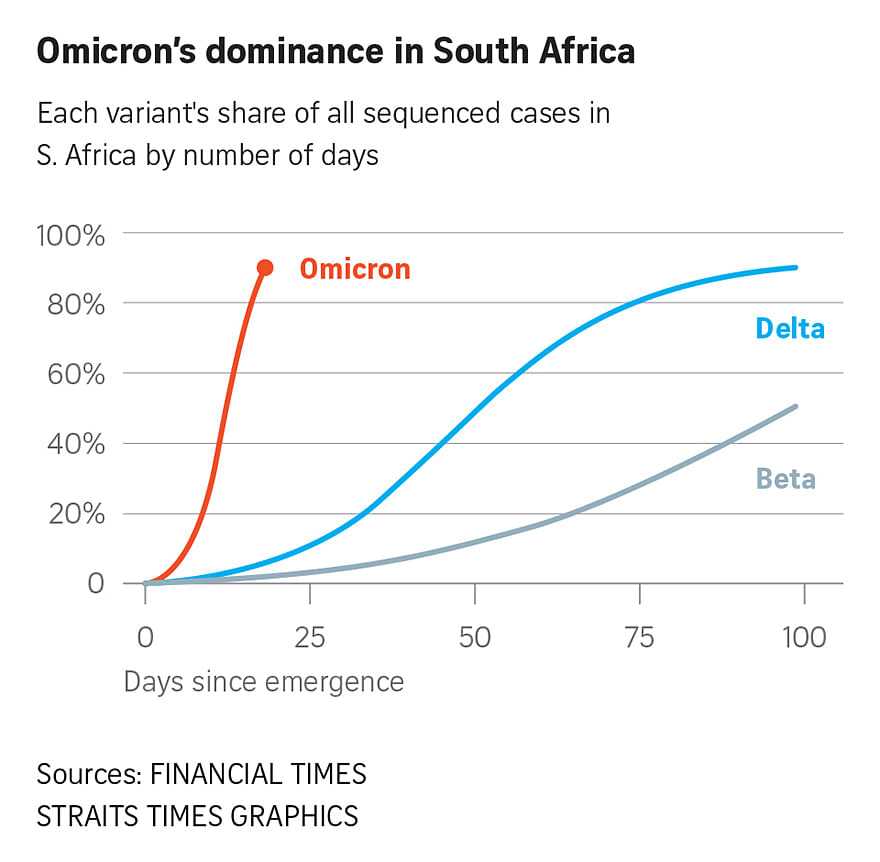
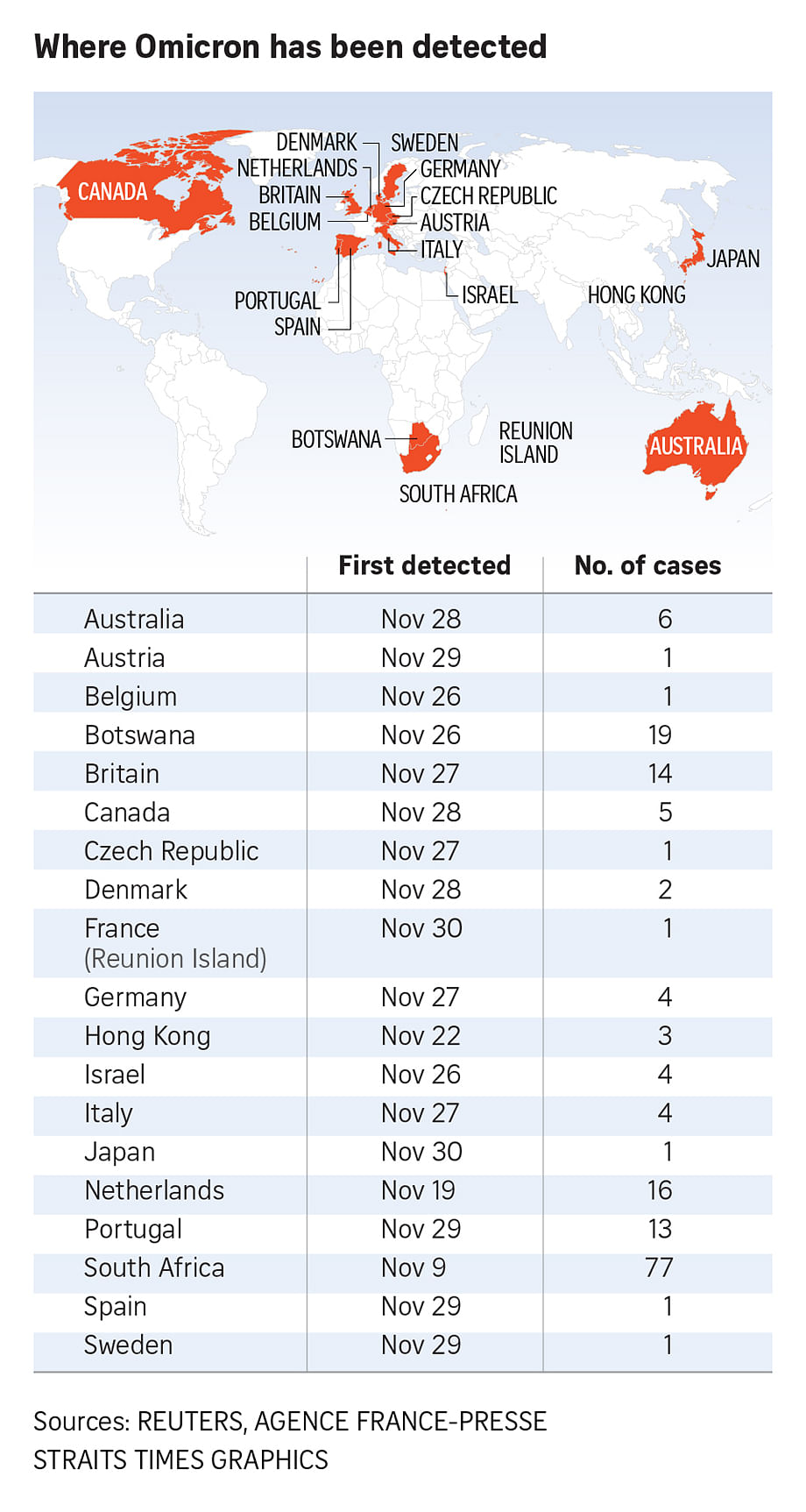
BRUSSELS (AFP) - Vaccines specially adapted for the new Omicron coronavirus variant could be approved in three to four months if they are needed, the head of the European Union's drug regulator said on Tuesday (Nov 30).
The decision on whether new shots are required would, however, have to be made by other bodies, European Medicines Agency executive director Emer Cooke said.
"Were there a need to change the existing vaccines, we could be in a position to have those approved within three to four months," Ms Cooke told a European Parliament committee.
"That's from the start of the time when they would start to change."
Her comments come after the head of US vaccine maker Moderna was quoted as saying that existing jabs will struggle against the heavily mutated Omicron.
Moderna has already said it is working on an Omicron-specific vaccine, as is US rival Pfizer.
Ms Cooke said EU regulators do not know yet whether the current vaccines remain effective against Omicron - which she said would take about two weeks to find out - or whether new ones are needed.
"A decision needs to be made first whether that's necessary, and that's not a decision for the European Medicines Agency," Ms Cooke said.
The agency has so far approved four vaccines for use for adults in the EU: Pfizer-BioNTech, Moderna, AstraZeneca and Johnson & Johnson.
A decision on a fifth, by US firm Novavax, is expected within a matter of weeks, Ms Cooke said.