Brazil trial finds China's Sinovac vaccine more than 50% effective
Sign up now: Get ST's newsletters delivered to your inbox
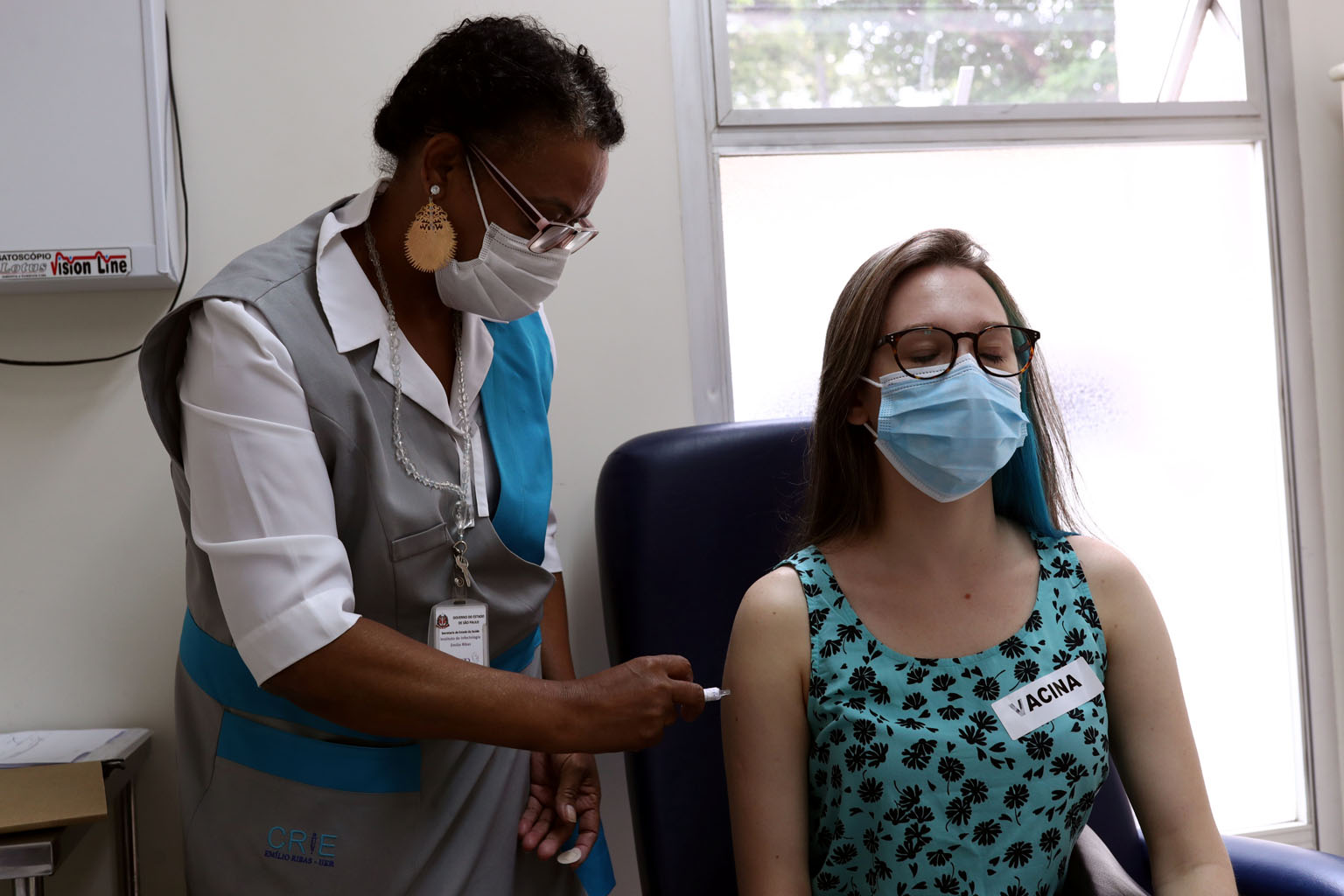
Ms Giovana Zanotto do Carmo receiving an injection as she volunteered in the trial stage of the vaccine developed by Chinese pharmaceutical firm Sinovac Biotech, at Emilio Ribas Institute in Sao Paulo, Brazil, on Dec 11.
PHOTO: REUTERS
RIO DE JANEIRO • A Covid-19 vaccine developed by Chinese pharmaceutical firm Sinovac Biotech was found to be more than 50 per cent effective in a Brazilian clinical trial, though researchers delayed releasing more information at the request of the company.
A 50 per cent efficacy rate is a minimum standard set by United States regulators for emergency authorisation of Covid-19 vaccines.
Messenger RNA vaccines from Moderna and Pfizer-BioNTech have produced far better results, reducing symptomatic Covid-19 cases by well over 90 per cent in trials.
Chinese vaccine developers have been slow compared with their Western peers in releasing efficacy data on their shots.
As millions of healthy people count on transparency in trials before taking a shot, the lack of more specific results from Sinovac's trial risks eroding confidence in vaccines from China.
Hong Kong has said residents will be allowed to choose which shot they want to take among several candidates that will likely include Sinovac.
The lack of transparency in reporting the Brazil trial results "is totally unacceptable", and would not pass muster in the US, said Dr Eric Topol, a clinical trials expert and director of the Scripps Research Translational Institute.
A spokesman said earlier this week that the company could disclose efficacy data only after they are reviewed by Chinese regulators.
The late-stage trial of Sinovac's vaccine in Brazil, involving about 13,000 participants, suggested that the shot is "safe and effective", the authorities at the Butantan Institute and from the state of Sao Paulo said.
They were asked not to disseminate the information until it was thoroughly reviewed in China as part of a contractual agreement.
"Our goal was for the shot to be more than 50 per cent effective," said Sao Paulo state's Health Secretary Jean Gorinchteyn. "A vaccine that reaches at least that is already cause for celebration."
The group that received the vaccine in Brazil's trial had no severe cases of Covid-19, and the main side effect reported was mild pain at the injection site, said Dr Dimas Covas, head of Butantan. Efficacy is above the threshold needed for a vaccine to be registered by Brazil's health regulator known as Anvisa, which still has to approve the shot.
Mr Gorinchteyn said Sinovac's review of the data is slated to take within 15 days or less, and should not delay the planned Jan 25 start of inoculations.
"Sinovac has several ongoing trials, and it's important (they give) the data consistency," Dr Covas said. "The company can't analyse data from the same vaccine using different criteria, and can't have three different efficacy rates for the same vaccine."
Sinovac is also running trials in Indonesia and Turkey.
Sinovac is betting on a successful vaccine to inoculate more people around the world, especially in developing countries such as Brazil that will have limited access to the Pfizer and Moderna shots.
Sinovac's shot is also potentially more suited to developing nations as it can be kept at normal refrigerator temperatures, compared with the vaccines from Pfizer-BioNTech and Moderna which need minus 70 deg C conditions for storage and transportation.
BLOOMBERG


