Local start-up launches animal-free cosmetics testing platform
Sign up now: Get ST's newsletters delivered to your inbox

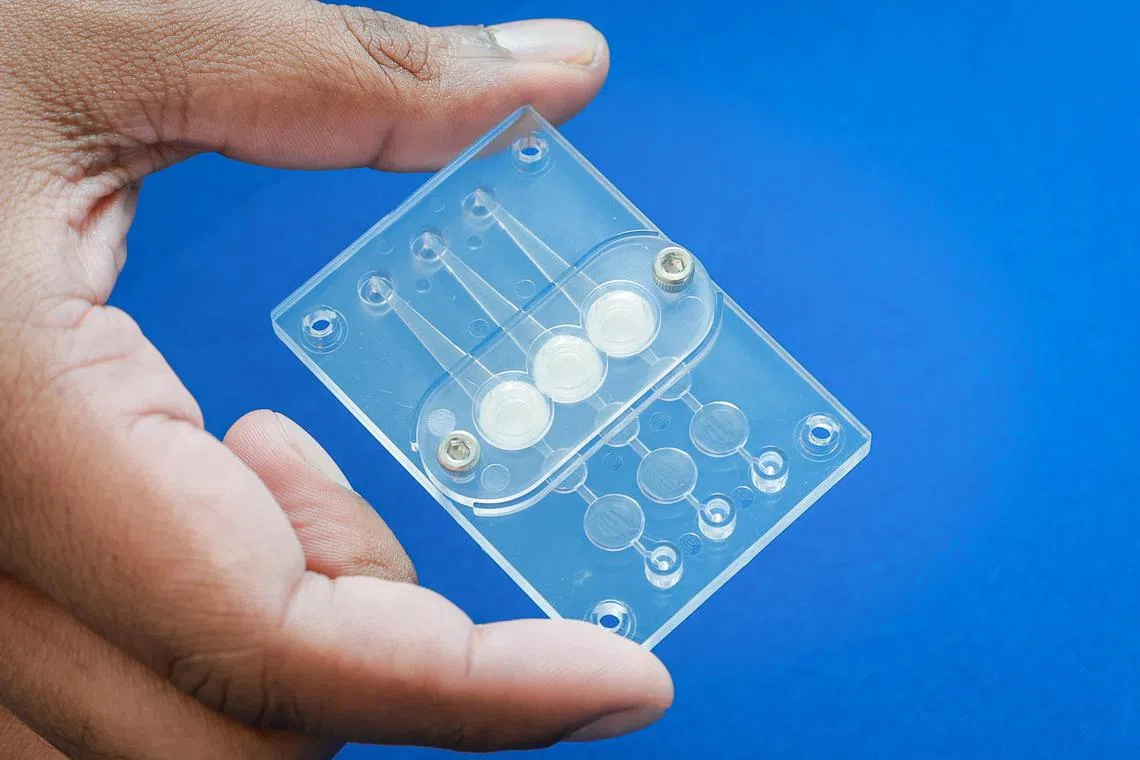
REVIVO BioSystems’ ReleGO, a 4D skin-testing platform, at a showcase event held at Star Vista, on March 2, 2023.
ST PHOTO: GAVIN FOO
SINGAPORE – Local biotech start-up Revivo BioSystems has developed a cosmetics testing platform which mimics the flow of blood under the skin, paving the way for more companies to move away from animal testing.
The in-vitro platform, launched on March 2, makes the company one of the front runners in the emerging field of “organ-on-chip” technologies, which seeks to model – on small chips made of plastic, glass or silicon – how human organs work.
These could make testing for drugs, foods and cosmetics in the lab more accurate and efficient.
In-vitro tests are performed in laboratories outside of human or animal bodies.
Revivo BioSystems’ platform has two devices that work together to evaluate the safety and efficacy of cosmetic products.
The first, known as the Revex chip, is a single-use plastic chip that has small channels that allow liquids to flow through. It has three chambers linked to these micro-channels, where human skin – taken, for example, from biopsies – can be placed and samples of the cosmetic product can be applied.
Synthetic substitutes for skin, created in laboratories, can also be used.
Four of these Revex chips can be put in a Relego collection device. As a liquid simulating human blood is pumped through these channels at body temperature (37 deg C), substances from the cosmetic product which have permeated through the skin sample and other proteins secreted by the skin cells dissolve in the fluid.
The fluid, and the substances it carries, is collected at preset time intervals. Its contents are analysed to show how the cosmetic product’s ingredients have interacted with the skin over time, to determine if the item is safe, or if it stands up to marketing claims.
Dr Massimo Alberti, chief executive and founder of Revivo BioSystems, said its dynamic platform is more accurate than traditional static cosmetic tests, which do not simulate the continuous flow of blood under the skin and can determine only the amount of permeated substances at a single point in time.
These static tests usually involve adding a test chemical to a reconstructed piece of human skin (made from cell cultures) that has the cosmetic product applied over it. As the chemical interacts with the skin, biological reactions occur and the substances formed can be analysed to determine if the skin reacted to the product in any way.
“Compared with other static systems which provide a very simplistic view of what is going on in the skin, our system allows us to look, over time, at what actually happens to the skin as it reacts to the cosmetic product,” said Dr Alberti.
Because the Relego collector is fully automated, the company said its platform reduces the man-hours needed to run in-vitro tests by up to 20 times.
It added that other cosmetic tests, such as those involving human volunteers where each is given a small dose of the product, still need to be performed.
With the enhanced accuracy and efficiency that the kit provides, Revivo BioSystems hopes that it will encourage more cosmetic companies to drop animal testing, which has already been banned in the European Union, South Korea and New Zealand.
In Singapore, the use of animals for testing cosmetic products is not prohibited, but it is regulated by the Animals and Birds (Care and Use of Animals for Scientific Purposes) Rules and the guidelines set by the National Advisory Committee for Laboratory Animal Research.
Though these regulations include the need to replace the use of animals by alternative methods as far as possible, reduce the numbers of animals used to an unavoidable minimum, and refine any procedures necessarily used to minimise the impact on animals, opponents argue that it is still cruel and immoral to subject animals to suffering just so that cosmetic products can be sold.
Revivo BioSystems’ technology is already patented in the US, Europe, China and Singapore. One of its first customers is speciality chemicals giant Evonik Industries, which has invested in the company and intends to use its platform to test its beauty and personal care products.
REVIVO BioSystems’ REVEx, a three-chamber microfluidic device, at a showcase event held at Star Vista, on March 2, 2023.
ST PHOTO: GAVIN FOO
Evonik’s chemicals are used in cosmetic products such as make-up, skin cleansers and skin creams.
Said Ms Shirley Qi, Evonik’s president for South-east Asia, Australia and New Zealand: “Revivo BioSystems’ kit will allow us to perform new dynamic testing methods. The automation takes human error out of the sampling, and provides a cost-effective way of carrying out screenings that are required in the approval procedures for new substances.”
Dr Alberti said Revivo BioSystems has signed deals with companies in the Fortune 500 list, which showcases the US’ biggest companies by revenue.
Revivo BioSystems’ launch of its “organ-on-chip” product follows Harvard University spin-off Emulate’s development of similar devices.
The university’s Wyss Institute for Biologically Inspired Engineering has used the chips in research for a variety of diseases and their treatments, including Covid-19, influenza and malnutrition.
In 2020, US-based market surveying firm QY valued the “organ-on-chip” industry at US$21 million (S$28 million), but this could grow to about US$220 million by 2025.
Dr Alberti said his company will continue looking for new applications for its platform, including the testing of drugs.
Singapore, a biomedical sciences hub in Asia, is already home to the manufacturing plants of some of the biggest pharmaceutical companies in the world, including GSK, Pfizer and Amgen.
Said Dr Alberti: “Skin is just the beginning. What we can do with skin, we can do with any other tissue which represents barriers where drug action occurs. The opportunities that the product offers are really rich.”


