S’pore scientists develop ‘nano-nets’ to trap, kill bacteria amid growing antibiotic resistance
Sign up now: Get ST's newsletters delivered to your inbox
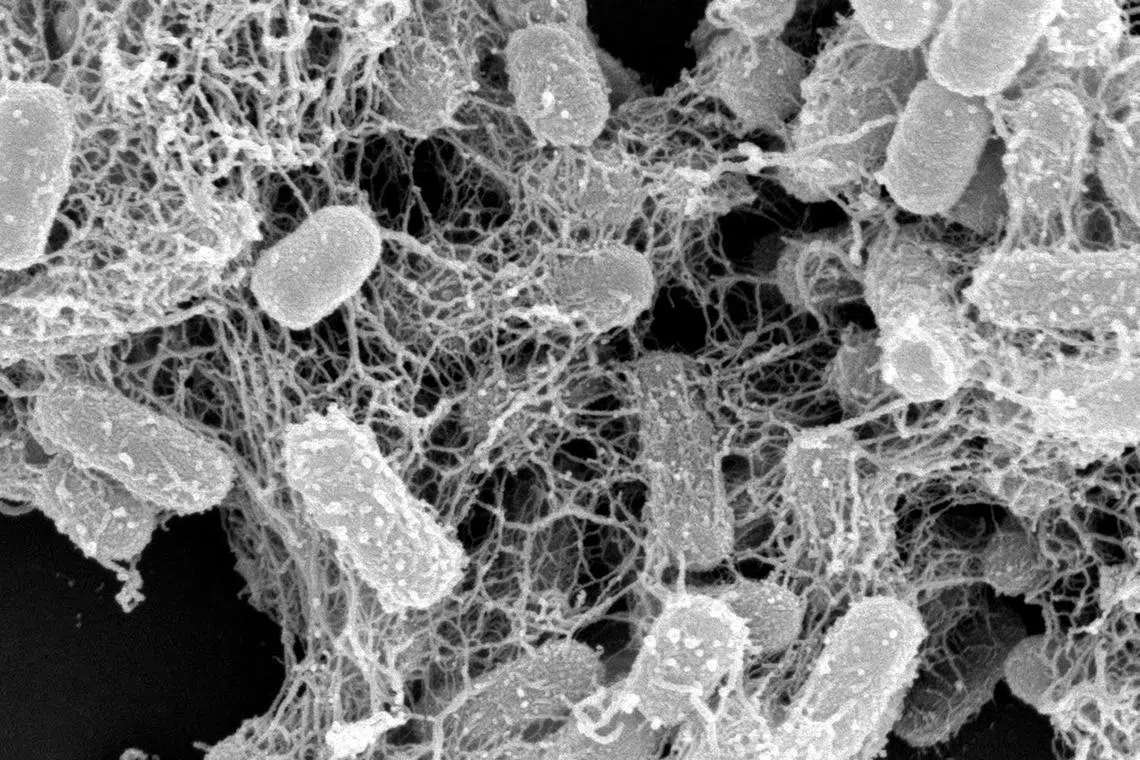
Antibiotic-resistant E. coli entrapped by peptide nano-nets. Antimicrobial resistance is projected to kill 10 million people by 2050.
PHOTO: NATIONAL UNIVERSITY OF SINGAPORE
SINGAPORE – With bacteria becoming increasingly resistant to even the strongest antibiotics, scientists around the world have been urgently trying to develop new drugs to prevent this “silent pandemic” from worsening.
Antimicrobial resistance is projected to kill 10 million people by 2050
However, any breakthroughs in the creation of new antibiotics appear to be some time away, and how long they will stay effective at fighting bacteria before germs evolve to become resistant to them is unpredictable.
Now, a team of 11 researchers has come up with a different approach to tackle the problem of antibiotic resistance: using microscopic nets to trap and kill bacteria in the body.
The researchers have successfully used these “nano-nets”, which are made out of molecules known as antimicrobial peptides (AMPs), to tackle E. coli and S. aureus in lab tests. These bacteria cause food poisoning and boils respectively, and have been found to be resistant to even the most potent antibiotics available today.
The nano-nets developed by the team mimic a natural body response to pathogens at various sites such as the small intestine, urinary tract and blood vessels.
In lab tests, the AMPs developed by the team formed the nets when they detected either lipopolysaccharide or lipoteichoic acid, which are two common chemicals found in bacterial cell membranes.
In the presence of the chemicals, the individual peptide molecules attached themselves to the bacteria and in turn attracted other peptides to link up with them, eventually forming an interlocking web – the nano-net – capable of entrapping the bacteria.
These peptides were also able to disrupt the bacterial membranes, effectively tearing apart and killing the germs.
Though the nano-nets have not been tested in humans, the researchers theorise that the trapping of bacteria in the body by the nets would make the germs more vulnerable to antimicrobial compounds secreted by the immune system – essentially augmenting the human body’s innate ability to combat bacterial infections.
Recreating these nano-nets synthetically to trap and kill antibiotic-resistant bacteria is not new, but the team’s work has helped to advance the field, said Associate Professor Rachel Ee of the National University of Singapore’s Department of Pharmacy, who co-led the team with her colleague from the same department, Associate Professor Rajamani Lakshminarayanan.
“In previous attempts to form synthetic nano-nets from AMPs to trap and kill bacteria, the AMPs could form only short, disjointed strands of molecules, which could not entwine the bacteria very well.
“By modifying the chemical compositions of the AMPs previously used, our team’s peptides could self-assemble into extensive, cross-linked nano-nets, which are more suitable for physically entrapping and immobilising bacteria cells.
“In addition, most of the synthetic peptide nano-nets that have been developed so far can only trap bacteria, whereas the modifications we made allowed our nano-nets to both trap and kill them.”
Prof Ee highlighted that the nano-nets had some advantages over antibiotics in their ability to kill bacteria.
“Bacteria are less likely to develop resistance to our nano-nets compared with new antibiotics, since our nano-nets physically entrap the bacteria instead of targeting specific proteins they contain, which is how antibiotics work. This means that the bacteria cannot just mutate and alter their proteins to evade detection by the nets, which is one of the ways germs become resistant to antibiotics.
“Even if the bacteria become resistant to the killing ability of the nano-nets, the nano-nets’ trapping effect alone can already boost the immune system’s capacity to eliminate them.”
Associate Professor Surajit Bhattacharyya of Nanyang Technological University’s School of Biological Sciences, who was not involved in the study, said the Singapore team’s novel mode of bacterial cell killing expands the horizon for developing new antimicrobials, which is “greatly needed”.
“Most AMPs are short strands of molecules that kill bacteria by disrupting cell membrane structures at the surface of bacterial cells,” he noted.
“In contrast, the current work by the researchers is based on the idea of tuning AMPs to form very tiny net-like structures that are efficient in trapping bacteria by causing them to clump together so that they can be more easily killed by the body’s immune cells.”

NUS Department of Pharmacy's Associate Professor Rajamani Lakshminarayanan, Associate Professor Rachel Ee and Dr Dai Thien Nhan Tram, part of the team that developed nano-nets capable of trapping and killing bacteria.
PHOTO: NUS
The team hopes that its invention will be able to contribute to the global fight against antimicrobial resistance. It plans to formulate the nano-nets into a hydrogel that can be injected directly into a part of the body where there is a bacterial infection, and further optimise the design of the nets for humans.
Said Prof Ee: “Faced with the rapid emergence of antimicrobial resistance globally, there is an urgent demand for innovative strategies to ‘outwit’ the microbes’ ability to evolutionarily adapt through mutations.
“We envision that our discovery will raise the standards and offer an optimistic outlook in the field, thus encouraging further developments in this promising yet underexplored biomaterial for combating antimicrobial resistance.”


