NUS Medicine team aims to start clinical trial of targeted therapy for deadly brain cancer in 2025
Sign up now: Get ST's newsletters delivered to your inbox

Dr Sarah Ho, 39, co-founder of AGeM Bio, demonstrating how the therapy (in syringe) should be stored and kept cool before it is used for a patient.
ST PHOTO: BRIAN TEO
Follow topic:
SINGAPORE – In the future, patients who suffer a relapse of the deadliest form of brain cancer may get a second shot at life with the help of a novel gene therapy developed by researchers from the National University of Singapore’s Yong Loo Lin School of Medicine (NUS Medicine).
The scientists behind the stem cell-based gene therapy are aiming to start a clinical trial for glioblastoma patients of National University Hospital (NUH) by late 2025.
Glioblastoma is an incurable and aggressive brain tumour. Once diagnosed, patients are typically left with about two years to live.
This targeted therapy – designed to kill aggressive tumours and activate anti-cancer immunity – builds on a similar drug that the NUS Medicine scientists first used to treat dogs and cats with terminal cancers
A vial of the treatment comprises human stem cells carrying cancer-killing genes that naturally gravitate towards tumours.
The team from NUS Medicine’s biochemistry department includes Associate Professor Too Heng-Phon, adjunct senior research fellow Sarah Ho and postdoctoral fellow Woo Jun Yung.
Prof Too’s team initially worked on the therapy to combat aggressive human tumours until a vet who heard about it contacted them in 2018.
The first version of the treatment was used on 65 dogs and cats between 2018 and 2022.
It had two parts – first, the modified stem cells were injected into the animal, and then off-the-shelf anti-fungal drugs were taken orally.
The researchers developed a technology to insert large amounts of yeast-based genes into the stem cells that would react with the anti-fungal drugs.
This reaction would allow the cells to produce a chemotherapy drug called fluorouracil (5FU).
The modified stem cells act like suicide bombers, loading the toxic 5FU around the tumour to kill it, said Dr Ho.
5FU is commonly used in chemotherapy, and can cause severe side effects such as diarrhoea, bleeding gums and a higher risk of infection.
But since the 5FU from the NUS Medicine therapy surrounds only the cancer cells, it does not cause serious side effects, though mild ones can occur, Dr Ho added.
The first version of the treatment focused on killing cancer cells.
Fifty-six of the 65 dogs and cats that received the treatment lived longer than expected, or had a better quality of life.
The second version has an additional ingredient that will enhance the recipient’s immunity. The stem cells are engineered to produce interferon beta, a substance naturally produced by cells.
“Interferon beta activates the body’s immune system, allowing the immune cells to attack the tumour so that you get a long-term suppression of the tumour,” said Dr Ho.
“All these point towards: You can’t just kill the cells. You also need to activate the immune system. If not, you will forever be trying to kill every single cell in the tumour environment,” she added.
Since 2023, 30 cancer-stricken dogs and cats have been given this improved treatment at some vet clinics.
The team has not done a formal study to analyse the 30 animals’ outcomes as it does not have enough patients yet.
All 95 animals underwent the treatment for free, as a form of compassionate care by NUS Medicine.
The current funding that the researchers have for the pets’ treatment will be used up by end-2024, said Dr Ho.
From 2025, pet owners will have to start paying for the therapy, though they will be charged below the commercial rate just to keep production going. The fees have not yet been decided with vet clinics and partners.
In January, Dr Ho and Dr Woo set up their biotechnology start-up AGeM Bio to scale up their animal and human cancer therapies.
In August, they will move to their new office and laboratory at the E7 building – an incubator for NUS technologies.

Dr Sarah Ho working with the modified human stem cells in a cell stack. These modified stem cells will be used for dogs and cats with terminal cancers.
ST PHOTO: BRIAN TEO
The improved therapy will be used for the brain cancer clinical trial for humans, which is expected to start in the third or fourth quarter of 2025.
The researchers did pre-clinical studies on mice with brain cancer to prepare for the first phase of the clinical trial at NUH. A week after the therapy was given, the brain tumours in some of the rodents had disappeared.
The team is currently doing more pre-clinical studies and is preparing a dossier to submit to the Health Sciences Authority. It will produce the treatment doses at the Advanced Cell Therapy and Research Institute, Singapore.
Stage one of the clinical trial will test the safety of the treatment on six to 15 patients with recurrent glioblastoma, that is, patients who have had their tumours return after previous surgery.
However, they have to be fit enough to undergo a second brain operation, said Clinical Associate Professor Yeo Tseng Tsai, a senior consultant in NUH’s division of neurosurgery.

The two NUS Medicine researchers and co-founders of AGeM Bio, Dr Sarah Ho (left) and Dr Woo Jun Yung (right), with Clinical Associate Professor Yeo Tseng Tsai from the National University Hospital’s division of neurosurgery.
ST PHOTO: BRIAN TEO
“Since it’s a safety trial, it may not be ethical to offer the new treatment for newly diagnosed patients. But for recurrent glioblastoma, there is no standard of care, and so we can do a research trial like this,” said Dr Yeo, who has been caring for glioblastoma patients for about 40 years.
For patients who relapse, the cancer cells could also be resistant to their existing chemo pill. The recurrence rate for glioblastoma is nearly 100 per cent.
For the clinical trial, patients will undergo another brain operation to remove as much of the cancerous tumour as possible without causing neurological damage.
Three millilitres of modified stem cells is then injected around the cavity with tumour remnants, done through 20 jabs. This is to maximise the remaining tumour’s exposure to the cancer-killing cells, said Dr Ho.
The patients will later take anti-fungal drugs orally.

Three millilitres of the modified human stem cells being harvested and collected into a syringe. The harvesting process is done by a machine at NUS Medicine’s department of biochemistry.
ST PHOTO: BRIAN TEO
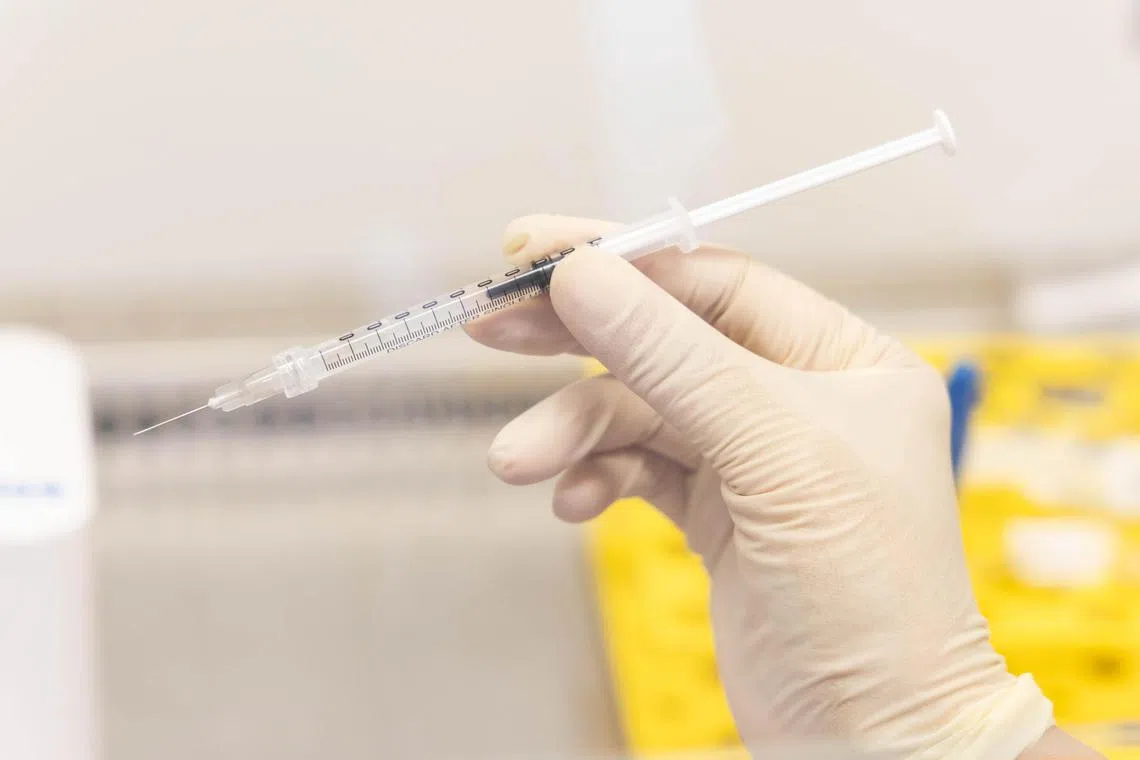
A sample of a syringe containing the modified stem cells which will be injected into the remaining tumour site 20 times.
ST PHOTO: BRIAN TEO
The surgical site will then be sealed with glue to prevent the stem cells from leaking.
If not enough suitable patients for the clinical trial can be found within NUH, patients will be recruited from Ng Teng Fong General Hospital or the National Neuroscience Institute.
Alongside his terminally ill brain cancer patients, Dr Yeo yearns for a successful treatment.
He noted that there are numerous new treatments being developed for glioblastoma globally, but “there’s so little headway in glioblastoma therapies around the world”.
“I’m not so optimistic that we will cure glioblastoma. But maybe we can convert it into a chronic disease. So instead of patients living 18 to 24 months, like they do now, maybe they can live five or 10 more years,” he said.
“That would already be a great achievement.”


