A*star spin-off Mirxes looking into tests for other cancers after success with kits for gastric cancer
Sign up now: Get ST's newsletters delivered to your inbox

(From left) Dr Wei Ying, Dr Zhou Lihan (seated), Mr Kenneth Tan, Dr Tang Yew Chung and Ms Sameema Begum, at M Diagnostics, on Dec 17, 2021.
ST PHOTO: DESMOND WEE
SINGAPORE - Long before Covid-19 struck, local biotech company Mirxes had, for seven years, been developing polymerase chain reaction (PCR) tests to detect cancer.
This allowed Mirxes to pivot quickly to making Covid-19 test kits when the need arose.
The first locally made Covid-19 test kit was developed within just three weeks.
The Fortitude Kit, developed together with the Agency for Science, Technology and Research (A*Star), came at the height of the Covid-19 pandemic in March last year.
Mirxes was founded in 2014, as a spin-off from A*Star.
Dr Zhou Lihan, Mirxes' co-founder and chief executive, said the ability to put the test kit together so quickly came on the back of decades of research - even when the term "PCR" was still unfamiliar.
PCR tests look for certain changes in one's gene, or for genetic materials in bacteria or viruses, such as Covid-19.
Prior to commercialising its technology, the team started its research in 2002, when it delved into the role of microRNAs in human cells - a type of genetic material that is essential in regulating gene expression.
"Ninety-five per cent of the human genome is made of microRNA, while 5 per cent is made of messenger RNA which teaches cells to make proteins. Both work in tandem to ensure that the body is in homeostasis, or a steady state. Changes in either the mRNA or microRNA could cause problems," said Dr Zhou.
For instance, most cancers, such as gastric cancer, produce abnormal levels of microRNA which are secreted into the bloodstream even at its early stages.
"So from there, we developed a PCR test, using microRNA as a biomarker to test for early-stage gastric cancer," he added.
The test kit - known as GastroClear - is taken as a blood test, and was approved for use here by the Health Sciences Authority in 2019.
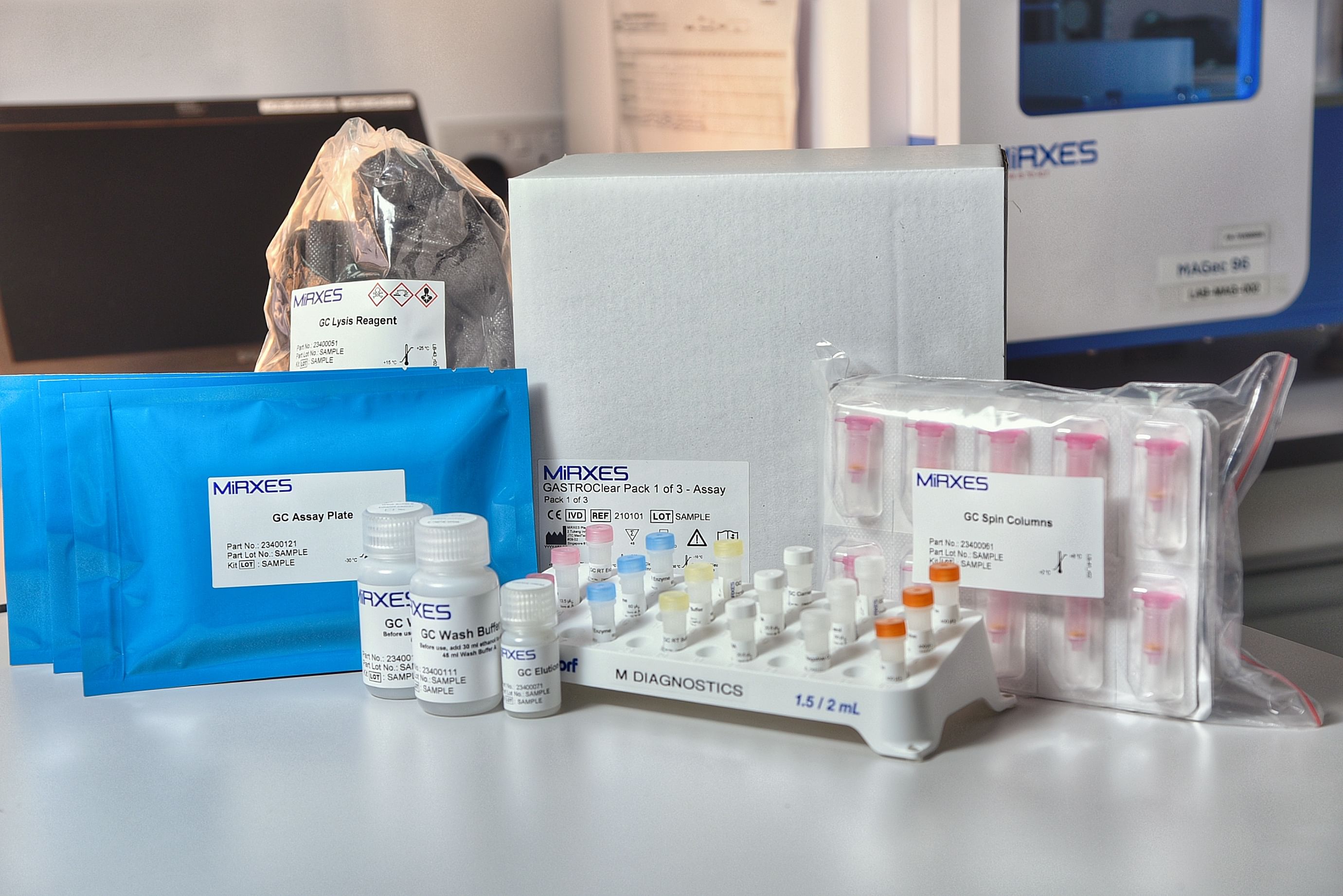
It has since been rolled out at eight hospitals and at multiple health screening centres and general practitioner clinics.
Gastric cancer is usually diagnosed through an endoscopy, a procedure perceived to be expensive and invasive, as it involves inserting a thin tube with a camera into the patient's mouth all the way to the stomach.
"We hoped that by introducing a blood test, more people can be screened for gastric cancer early, and begin treatment at an early stage," said Dr Zhou.
In Singapore, gastric cancer is the fifth cause of cancer deaths in men and the sixth in women, claiming around 300 lives yearly.
It is also the third leading cause of cancer deaths worldwide, as it is often detected at the later stages, making it difficult to treat.
Plans are also in place to take GastroClear to other countries in South-east Asia, followed by China and Japan, where there is a high prevalence of gastric cancer.
Amid the push to commercialise its cancer test kits globally, the company in July also secured US$87 million (S$119 million) in funding, bringing its current valuation to more than US$500 million.
Apart from gastric cancer, a study is also under way to test the efficacy of Mirxes' microRNA test kits for the early detection of liver cancer - the third-deadliest cancer among men, and fourth among women here.
Mirxes is also looking at the possibility of coming up with a diagnostic test for lung cancer, which can be challenging as the cancer has many different sub-types, and has vastly different disease outcomes.
"Therefore, we have to find a biomarker that is consistent throughout each lung cancer subtype," said Dr Zhou.


