HSA removes over 3,000 online listings for illegal health products including injections, antibiotics
Sign up now: Get ST's newsletters delivered to your inbox

Prescription-only acne treatment gel (left) and cream for skin allergies and itching were found listed for sale online.
PHOTOS: HSA
SINGAPORE - The Health Sciences Authority (HSA) has removed more than 3,000 illegal health product listings from local e-commerce and social media platforms in a bid to clamp down on the illegal sale and supply of such products.
In what the HSA described in a Nov 27 statement as a “first-of-its-kind large-scale collaboration with online platform administrators”, a total of 3,336 illegal health product listings were removed.
The authority also issued 1,471 warnings to sellers during the operation that took place from Sept 23 to Oct 23.
The eight platforms that participated in the operation were Amazon Singapore, Carousell, eBay Singapore, Facebook, Lazada, Qoo10, Shopee and TikTok.
“The joint operation to clamp down on the illegal sale and supply of health products sold online illustrates HSA’s commitment to protect consumers from products which are substandard or counterfeit. These products may be unsafe and carry a significant risk of harm
In September, a 32-year-old man was fined $266,500 after being convicted of possessing and supplying 126 types of cosmetic products that were found to be counterfeit – the largest fine ever imposed for selling counterfeit cosmetic products, said HSA.
He had intended to sell these counterfeit cosmetic products through multiple accounts he had created on Lazada.
Another case in August saw a 48-year-old woman fined $19,000 and jailed for two weeks for supplying unregistered health products during her home-based aesthetic services, as well as promoting her services on social media.
HSA seized 51 types of health products including dermal fillers and vials of lidocaine carbonate injection from the woman’s home.
In February, a 30-year-old woman was fined $18,000 for selling an unregistered skin cream known as Star Cream on several local e-commerce platforms.
HSA prosecuted the woman after investigating a “serious adverse event” reported in a four-month-old infant who was diagnosed with Cushing’s syndrome after the use of the cream, which his mother had purchased online. Cushing’s syndrome is a hormonal disorder that leads to excessive production of the stress hormone cortisol.
The cream was tested by HSA and found to contain clobetasol propionate, a potent steroid, and ketoconazole, a medicine for fungal infections.

An infant developed Cushing’s syndrome after using Star Cream.
PHOTOS: HSA
Prescription medication, sexual enhancement products
Aesthetic enhancement products formed the majority – about 48 per cent – of the removed listings, with 1,611 listings.
These products included do-it-yourself beauty injectable kits skin whitening eczema
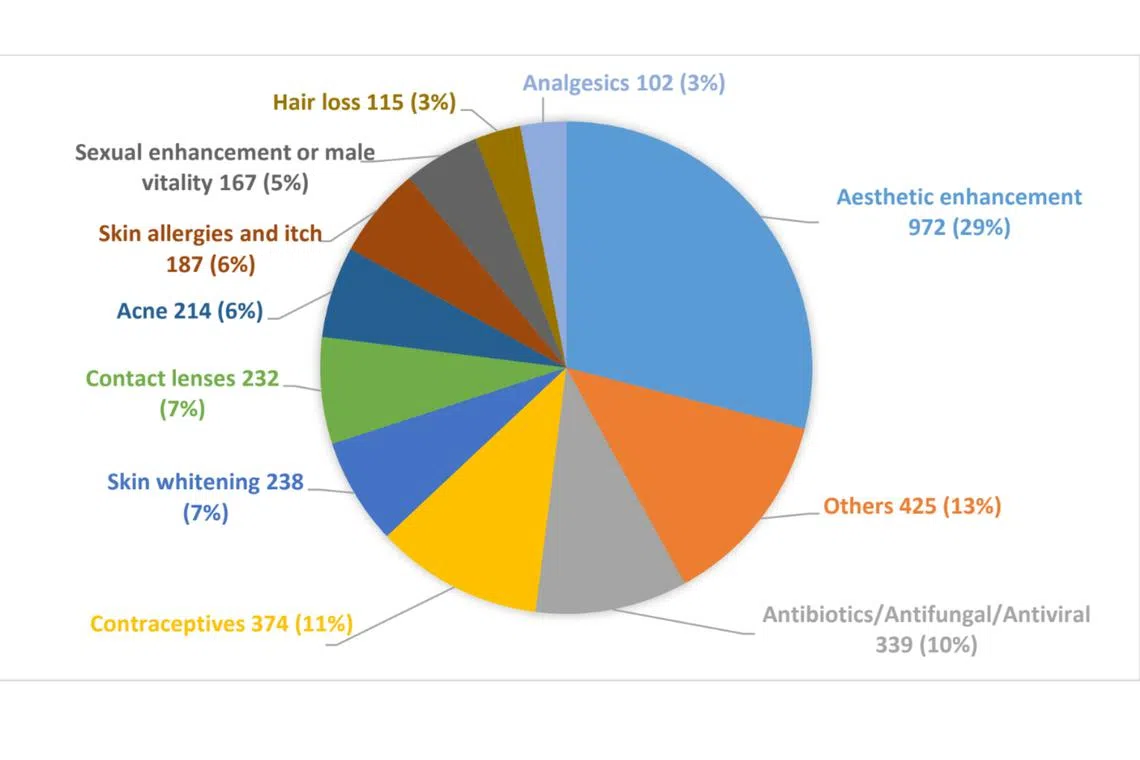
Types of product listings detected and removed from local e-commerce and social media platforms by the Health Sciences Authority.
PHOTO: HSA
HSA singled out do-it-yourself aesthetic beauty injectable kits comprising dermal fillers or botulinum toxin injections in particular, which it said are health products that require its approval before they can be supplied and should be administered by qualified medical practitioners.
The authority had cautioned the public in 2023 against the use of such kits
It said that inappropriate or incorrect administration of injections, coupled with the potential risk of injecting unknown substances in the face, can result in complications such as anaphylactic shock, severe infections, blockage of blood vessels leading to tissue death, also known as necrosis, blindness and stroke.
An online listing for an aesthetic enhancement kit with the prefilled syringe and needle.
PHOTO: HSA
The remaining listings removed included contraceptives (11 per cent), antibiotics or antifungal or antiviral products (10 per cent), contact lenses (7 per cent), sexual enhancement and male vitality products (5 per cent), hair loss products (3 per cent) and analgesics for pain relief (3 per cent).

An online listing for a prescription-only contraceptive medicine.
PHOTO: HSA
“Contact lenses are medical devices, and they must be prescribed and dispensed only by registered optometrists or contact lens practising opticians. Consumers who use these products from online sources face risk of severe adverse reactions
In May, a 24-year-old woman who bought contact lenses online said she began feeling a stinging sensation in her eyes when she wore them. She described her eyes as “red and felt as though they were on fire”
Her eyes were found to be infected due to the lenses, and she was prescribed antibiotics and special eye drops to treat the problem.

An online listing for contact lenses.
PHOTO: HSA
Prescription- and pharmacy-only medicine made up about 40 per cent of all listings removed, said HSA, adding that such medicine should be obtained from doctors or from pharmacists with a doctor’s prescription.
It warned in November 2023
It is illegal to sell unregistered aesthetic enhancement products and dermal fillers
Anyone found guilty of supplying such health products can be imprisoned for up to three years and/or fined up to $100,000.
HSA said it takes a serious view against those engaged in the illegal sale and supply of these products, and will take strong enforcement action against such individuals.



