HSA recalls 3 versions of diabetes drug metformin amid global testing for carcinogen
Sign up now: Get ST's newsletters delivered to your inbox
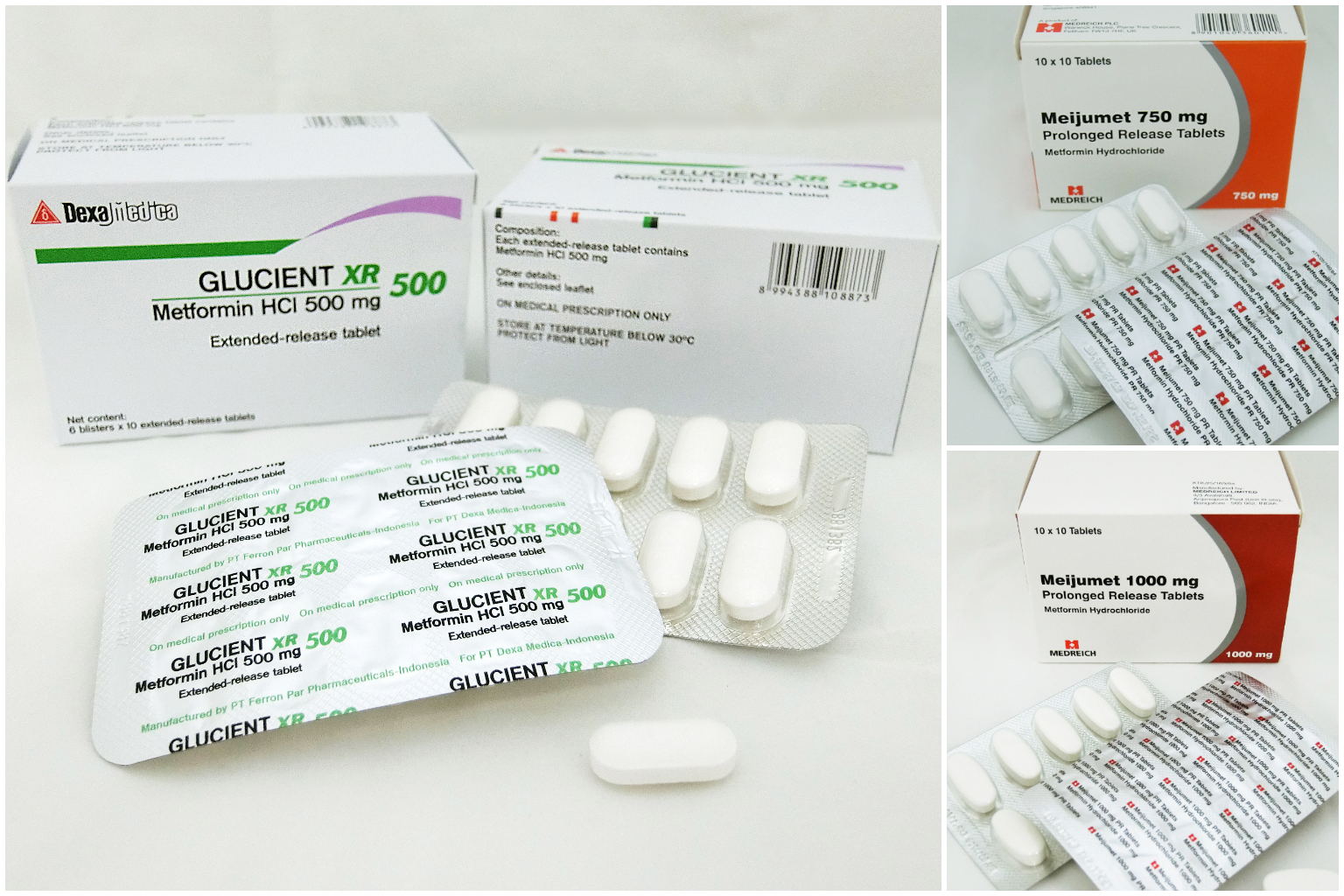
The affected medications are batch number 2881382 of Glucient XR Tablet 500mg, and all batches of Meijumet Prolonged Release Tablet 750mg and 3 Meijumet Prolonged Release Tablet 1000mg.
PHOTO: HEALTH SCIENCES AUTHORITY
Follow topic:
SINGAPORE - Three versions of a diabetes medication are being recalled here after they were found to contain unsafe levels of an impurity that can potentially cause cancer.
The Health Sciences Authority (HSA) tested all 46 locally marketed metformin medicines and the remaining 43 are not affected.
The recall comes even as health authorities worldwide are checking if the impurity, carcinogen earlier found in heart and gastric drugs, might also be found in unsafe levels in diabetes drugs that are widely used.
HSA said on Wednesday (Dec 4) that three metformin drugs were found to contain trace amounts of a nitrosamine impurity, called N-nitrosodimethylamine (NDMA), which are above the internationally acceptable level.
NDMAs are classified as potential carcinogens for humans.
The authority tested all 46 locally marketed metformin medicines and the remaining 43 are not affected.
As a precaution, HSA said that it is recalling the three affected drugs, which are used to control high blood sugar levels in diabetic patients.
The recalled drugs are: one batch of Glucient XR Tablet 500mg, supplied by Glorious Dexa Singapore; and all batches of Meijumet Prolonged Release Tablet in 750mg, and 1,000mg versions, supplied by Pharmazen Medical.
The authority said that the risk to patients who have been taking the affected medicines is "very low". This is because the potential risk of nitrosamines is associated with long-term use of the affected drugs, and the medicines have only been supplied here for a short period of time - since last year.
Patients taking the affected metformin medicines are advised not to stop treatment on their own, as doing so suddenly will raise blood sugar levels, which may pose a greater health risk than the trace amounts of NDMA in the affected medicines.
It has also advised healthcare professionals to contact their patients who are taking the affected drugs and arrange for an exchange of their medicines as soon as possible.
Patients who are concerned about their current treatment should speak to their doctor or pharmacist.
Besides being a potential human carcinogen, the NDMA nitrosamine impurity found in the diabetes drugs is also known to cause cancer in animals, Bloomberg reported on Thursday.
NDMA can be found in food or the environment. They are commonly found in low levels in processed food - including pickled vegetables, salted fish, processed meat products such as bacon and sausages - and in polluted air.
Nitrosamine impurities have recently also been found to be formed unexpectedly during the manufacture of some medicines.
Recalls were done worldwide for affected products found to contain these impurities above acceptable levels.
HSA said that acceptable levels of nitrosamines are set in one-billionth of a gram. This is based on what is considered as reasonably safe if a patient continues to take the affected medicine every day for a lifetime of 70 years.
On Wednesday, the United States Food and Drug Administration (FDA) said that it is testing samples of metformin sold in the US for NDMA, Bloomberg reported. The agency will recommend recalls of the medication as appropriate.
The European Medicines Agency said on the same day that companies should test for high levels of NDMA in metformin. No dangerous levels have been detected in European Union supplies to date.
Poland's health minister, Lukasz Szumowski, said earlier that officials were scrutinising metformin-containing drugs but that no products would be pulled from the market as NDMA had been found in only trace amounts.
HSA is working with the companies supplying the affected medicines and international regulatory agencies to verify the causes of the contamination, and identify the measures to address the issue.
Consumers can contact HSA on 6866-3538 or e-mail contact_hprg@hsa.gov.sg if they have more questions.

