NTU researchers develop new way to recycle plastic that leaves minimal carbon footprint
Sign up now: Get ST's newsletters delivered to your inbox

Associate Professor Soo Han Sen, who leads the project, holding up a bottle of solution with dissolved plastic and vanadium catalyst.
PHOTO: NTU
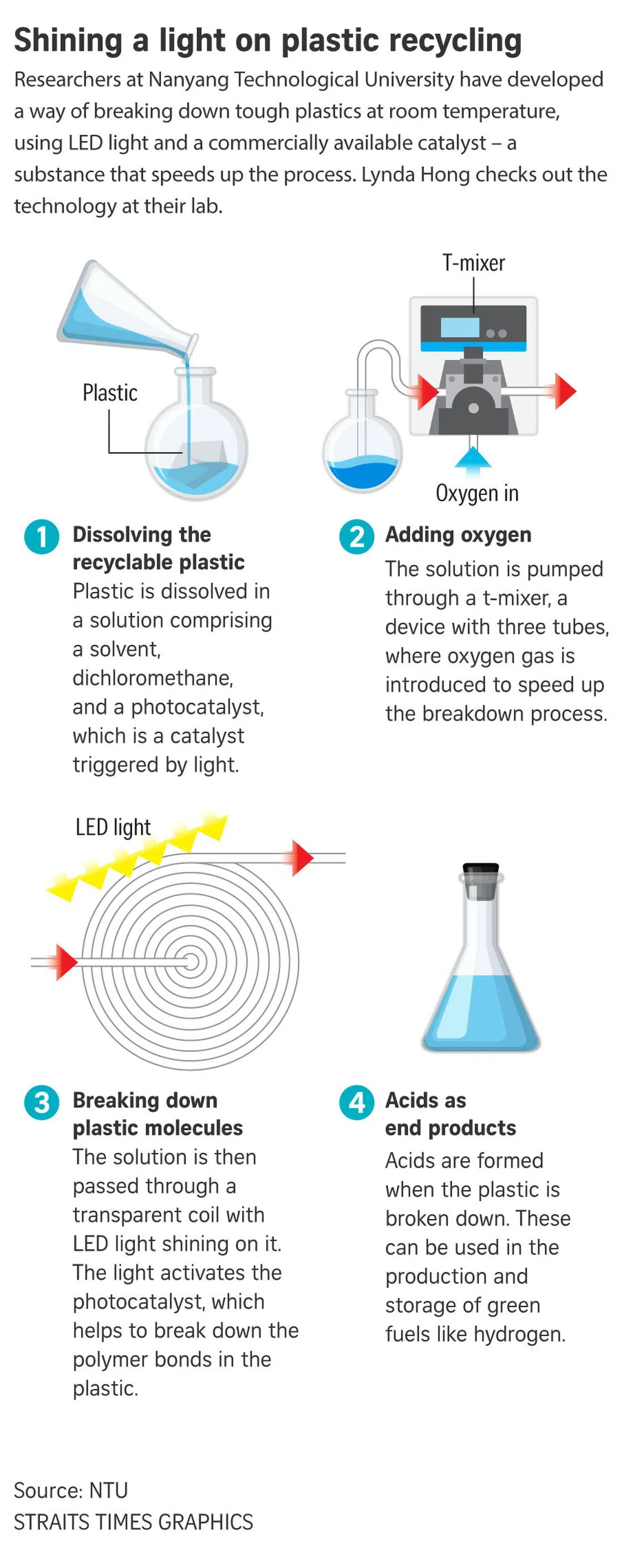
SINGAPORE - Plastic bags and takeaway boxes, styrofoam boxes and even PVC sewage pipes could be chemically recycled more efficiently to leave a smaller carbon footprint, in a new process developed by scientists from Nanyang Technological University (NTU).
It uses light and an easily available commercial photocatalyst – a substance that speeds up chemical reactions – to break down the stubborn polymer bonds of plastics.
Acids formed during this new process can be used in the production of green fuels such as hydrogen.
Associate Professor Soo Han Sen, who led the project, called the process “upcycling” rather than recycling.
Recycling, he said, has mostly been used to refer to mechanical recycling, where the durability of the plastic is reduced.
Mechanical plastic recycling is the process of recovering waste through sorting, washing, drying, grinding and re-granulating.
The final processing step is compounding, after which the recycled plastic material can be introduced into production processes.
By contrast, in the technology developed by NTU scientists that is currently in the process of being patented, formic acid, acetic acid and benzoic acid can be produced for use in making other chemicals for fuel cells and liquid organic hydrogen carriers (LOHCs).
As hydrogen is rarely found in a gaseous state, LOHCs can absorb hydrogen for safer transport before being burned to generate energy.
The research team is currently seeking industry collaborators to commercialise the technology.
Prof Soo said that the energy sector is exploring how LOHCs can play critical roles in clean energy development, given their ability to store and transport hydrogen gas more safely.
Currently, the derived chemicals from this new way of recycling would cost more than if they were produced from fossil fuels, said Prof Soo.
The technology could potentially use different photocatalysts to produce higher-value speciality chemicals for fragrances and paints. Photocatalysts are materials that change the rate of a chemical reaction when they are exposed to light.
More research and development is under way to make this technology more efficient, so that in a commercial setting, tonnes of plastic waste can be processed within a few hours or up to a day.

Associate Professor Soo Han Sen (right) with (from left) Dr Lyu Maoping, Dr Kong Xin Ying and PhD student Chan Wei Xin holding various types of plastics, an LED lamp and the vanadium catalyst and mixed plastic solution.
PHOTO: NTU
Currently taking up to six days to process plastic waste, the new chemical recycling tech uses organic solvent dichloromethane to dissolve the plastic and disperse the polymer chains.
The photocatalyst is then introduced into the solution before being pumped through transparent tubes in a flat spiral for oxygen and LED light to break down the plastic. No extra heat is required, as the solution of the photocatalyst can react at room temperature.
While the carbon footprint has yet to be quantified, Prof Soo is confident that the carbon emissions from this method will be much lower than those from conventional pyrolysis and mechanical recycling.
This is because pyrolysis usually uses high heat to treat the plastic waste to become fuel for burning, said Prof Soo.
“In pyrolysis, you’re putting in energy to turn it into fuel so that you burn it later. So that adds carbon dioxide to the atmosphere.
“If we consider the life cycle of the recycled plastic from pyrolysis, that’s actually even worse than incineration. With incineration, you directly burn the plastic to produce electricity.”

A close-up of the continuous flow set-up, where the dissolved plastic and vanadium catalyst solution is exposed to light from LEDs and transformed into acids that are useful to make fuel cells or hydrogen energy storage.
PHOTO: NTU
This research, which is under NTU’s Sustainable Plastics RepUrposing for a Circular Economy project, also considered the impact of carbon emissions from plastic waste.
While biodegradable plastics can generally be digested by microbes, rendering them less harmful to the natural environment, the carbon footprint could be much larger than from synthetic plastics, said Prof Soo.
“Plastic waste has actually been a good form of carbon storage,” he said.
Citing studies on plastic waste management which found that about 4.9 billion tonnes had accumulated in landfills and the natural environment by 2015, Prof Soo estimates that three to four times more carbon dioxide could have been released into the atmosphere by now, based on the chemical makeup of plastic.
“In other words, we would have produced an extra 15 to 20 billion more tonnes of carbon dioxide if all of the plastic had been biodegradable.
“And we would have easily exceeded 1.5 deg C,” he said, referring to the international agreement to limit greenhouse gas emissions so that global warming can be limited to no more than 1.5 deg C above pre-industrial levels.