Cordlife will not be allowed to collect, test, process and store new cord blood from Nov 26
Sign up now: Get ST's newsletters delivered to your inbox
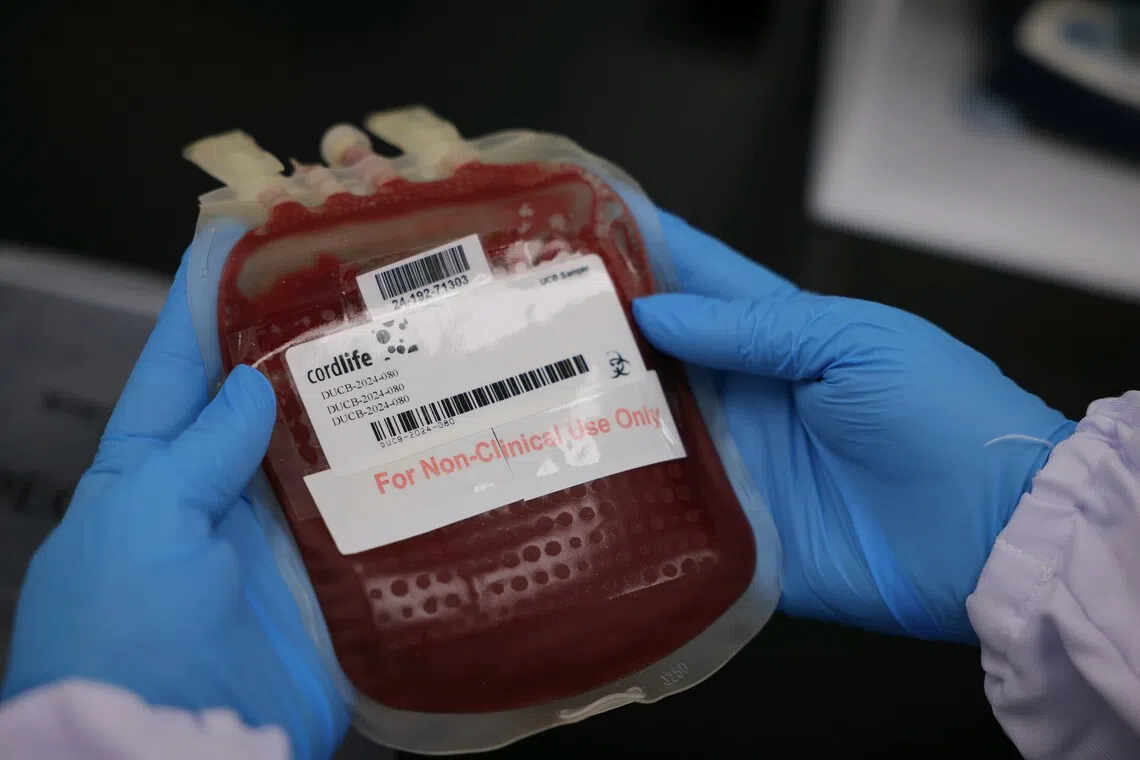
Cordlife will now be allowed only to maintain the storage of existing cord blood units.
PHOTO: ST FILE
- Significant lapses were found in a midpoint audit by MOH.
- The MOH found failures in governance, incident reporting and cord blood unit processing.
- Cordlife must replace its clinical governance officer.
AI generated
SINGAPORE – Beleaguered cord blood banking company Cordlife will not be allowed to collect, test, process and/or store new cord blood with effect from Nov 26.
It will only be allowed to maintain the storage of existing cord blood units (CBUs), and perform limited actions like facilitating their transfer to other cord blood banks and disposing of existing CBUs if clients instruct it to do so.
The regulatory action from the Ministry of Health (MOH) comes after “significant lapses” were discovered during its midpoint audit of Cordlife in July.
MOH issued a notice to Cordlife on Sept 29 on the intention to suspend its licence. The company then made written representations to the ministry.
MOH said that after careful consideration of Cordlife’s representations, it assessed that the company had not adequately addressed the concerns raised by the ministry during its midpoint audit to continue providing its cord blood banking service in a safe, clinically and ethically appropriate manner, and would need time to satisfactorily address the outstanding issues.
The suspension of collection, testing, processing and storing of new CBUs will remain in force even if Cordlife’s licence is renewed for a year in January 2026, and until the company demonstrates the ability to consistently meet the regulatory requirements for its cord blood banking services.
Cordlife first received a six-month suspension
It was allowed to resume operations in a calibrated manner
Cordlife’s licence was renewed for one year
However, the midpoint audit found that Cordlife failed to maintain its compliance with various regulatory requirements, including governance, incident reporting and management, as well as processes for collection, testing and processing of new CBUs.
MOH said in a briefing on Sept 29 that previous improvements like the establishment of standard operating procedures (SOPs) were still in place, but high staff turnover meant newer employees were unable to demonstrate that they understood the SOPs.
MOH highlighted that one of the key leaders in Cordlife – an unnamed clinical governance officer (CGO) – was found to have failed to provide proper oversight and guidance.
This led to process failures in the collection, processing and testing of about 160 new CBUs that Cordlife collected since January 2025.
Lapses included CBUs that were gradually frozen for storage and did not reach the optimal temperatures but continued to be stored.
There was also no evidence that proper investigations were conducted to determine if these deviations could have damaged the stem cells.
The CGO gave inappropriate advice that these were not issues of concern, resulting in an unknown number of similar incidents not being reported or investigated.
There were also cord blood collection bags exposed to inappropriate temperatures being used without validating if these would affect the quality of the CBUs collected.
On Nov 26, MOH reiterated its earlier directions to Cordlife to replace its current CGO and review all lab records of about 160 CBUs collected since January.
“MOH will continue to closely supervise Cordlife’s rectification of its various lapses, and will not hesitate to take further regulatory action should further non-compliances be found,” said a ministry spokesperson.
Cord blood contains stem cells that can be used to treat blood diseases and some cancers, such as leukaemia and lymphoma, should the baby develop these illnesses later in life.
The Straits Times has contacted Cordlife for comments.



