Asian Insider
Promising pipeline of Covid-19 vaccines in India, but affordability is key
Sign up now: Get ST's newsletters delivered to your inbox

A farmer receiving the Covishield vaccine in a field on his farm, during a door-to-door vaccination drive in Gujarat, India, last month.
PHOTO: REUTERS
Follow topic:
BANGALORE - When Indian Prime Minister Narendra Modi took a shot of the country's first indigenous Covid-19 vaccine on March 1, he said: "Remarkable how our doctors and scientists have worked in quick time to strengthen the global fight against Covid-19."
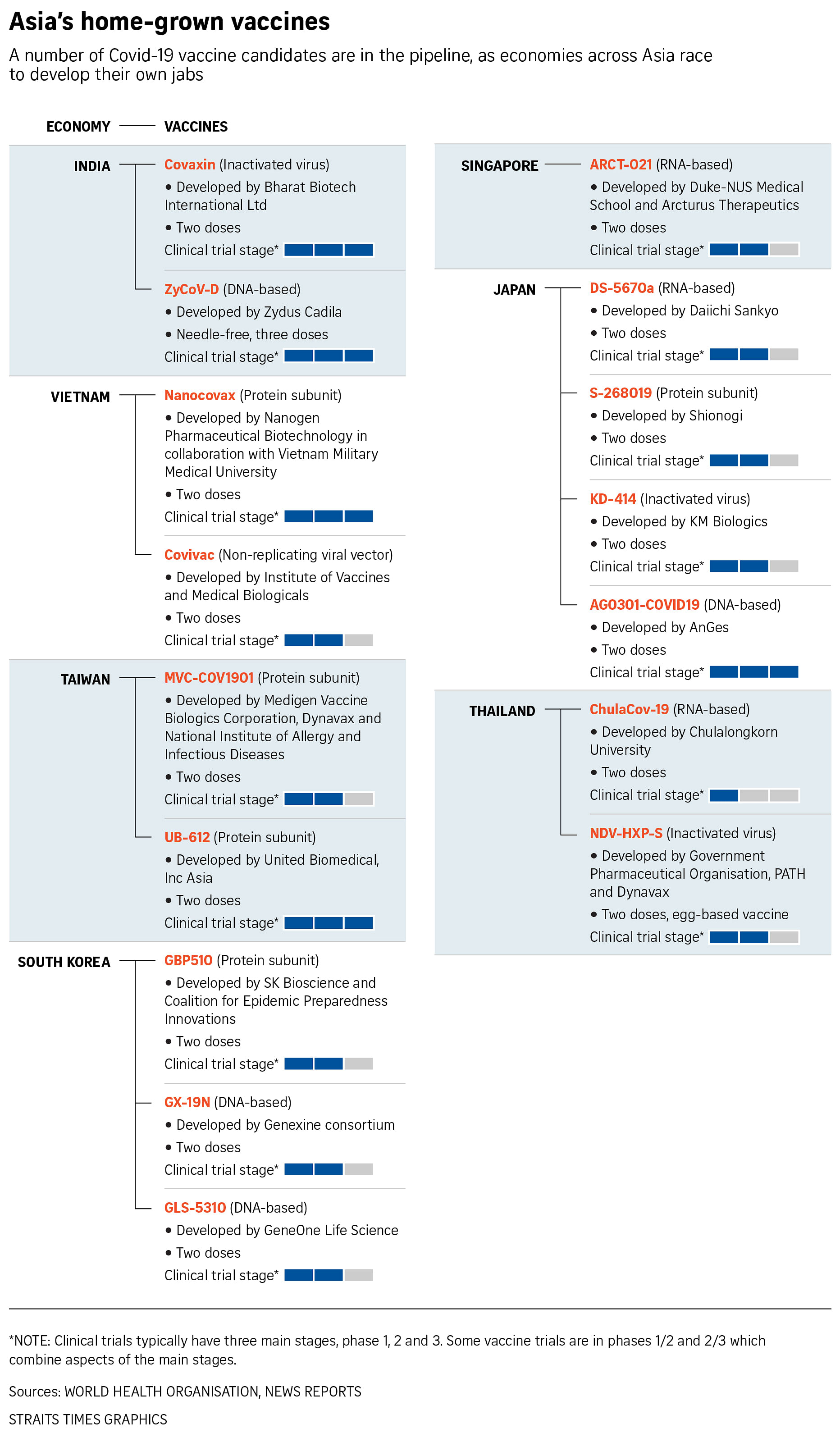
Three months earlier, Covaxin, developed by Bharat Biotech International using the whole inactivated coronavirus, had received emergency approval from India's drug regulator - even before the key third phase of its human clinical trial proved the extent of its efficacy against the coronavirus.
It was rolled out along with the Oxford-AstraZeneca vaccine manufactured by the Serum Institute of India, known as Covishield, in January.
Since then, 12 million doses of Covaxin have been administered. The efficacy data, still not peer reviewed, is said to be 77.8 per cent against symptomatic infections.
India is racing to develop more indigenous Covid-19 vaccines to inoculate its 1.3 billion population quickly, and also for export.
It is the world's primary maker and exporter of affordable vaccines for diphtheria, tetanus, human papillomavirus and hepatitis B.
India now has at least 15 domestic Covid-19 vaccines in different stages of development.
A needle-free three-dose vaccine by Zydus Cadila is in the third phase of its clinical trial. Called ZyCov-D, it is the world's first plasmid DNA vaccine.
It uses a genetically engineered, non-replicating version of a type of DNA molecule coded to make the spike protein of Sars-CoV-2. It is also being tested on those aged 12 to 18.
Biological E is working on an antigen, and Gennova Pharmaceuticals on an mRNA vaccine, in collaboration with American companies.
Ms Malini Aisola, co-convener for the watchdog All India Drug Action Network, said "a promising pipeline" of local vaccines could become available in the next few months or more likely next year, but it was important for India to "scale up manufacturing and increase affordability of the vaccines".