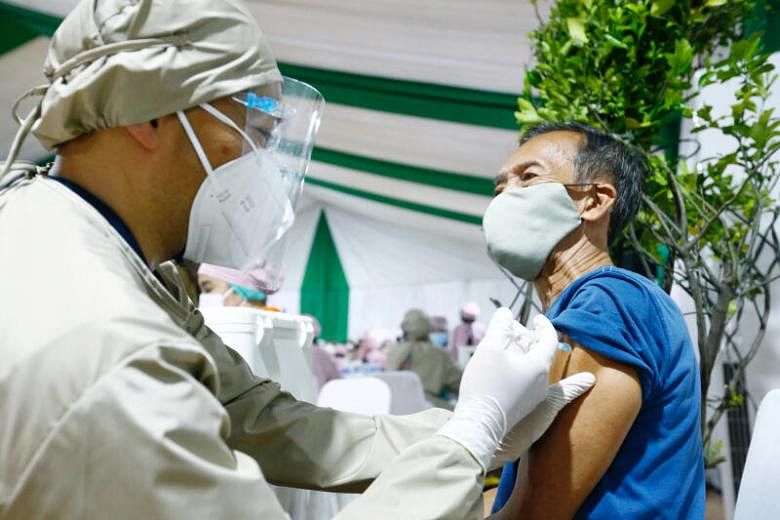
JAKARTA (REUTERS) - Indonesia will delay the administering of AstraZeneca's Covid-19 vaccine due to reports of blood clots among some recipients in Europe and would await a review from the World Health Organisation (WHO), its health minister said on Monday (March 15).
The European Medicines Agency has said there is no indication that the events were caused by the vaccination, a view echoed Friday by the WHO, while AstraZeneca said on Sunday its review has shown no evidence of an increased risk of blood clots.
"To be conservative, the food and drug agency delayed implementation of AstraZeneca (vaccine) as it awaits confirmation from the WHO," health minister Budi Gunadi Sadikin told a parliamentary hearing on Monday.
Indonesia received 1.1 million doses of the AstraZeneca vaccine via the Covax vaccine-alliance scheme this month and is set to receive some 10 million more in the next two months.
Thailand, which became the first country outside of Europe to delay use of the AstraZeneca shot on Friday, plans to start using the vaccine on Tuesday, officials said, with the prime minister and his cabinet the first to receive it.
The decision will leave Indonesia with just one approved vaccine, developed by China's Sinovac Biotech, for use in its nationwide vaccination drive. Its immunisation programme started in January and aims to reach 181.5 million people within a year.
South-east Asia's biggest country has been grappling with the worst outbreak in the region, having recorded more than 1.4 million infections and 38,500 deaths.
It expects to receive 20.2 million doses of Covid-19 vaccines developed by Moderna and China's Sinopharm from the second quarter to use in a private vaccination scheme, Mr Honesti Basyir, CEO of state pharmaceutical firm Bio Farma, said on Monday.
Indonesia authorised one of the world's first private vaccination programmes last month to run alongside its national drive, enabling firms to buy state-procured vaccines for their staff in the country.
While the plan is expected to speed up the pace of inoculation in the world's fourth most populous country, some health experts have warned it could worsen inequity.
Mr Honesti told a parliamentary hearing on Monday that it had ordered 15 million doses from China National Pharmaceutical Group (Sinopharm) and 5.2 million from Moderna. The Sinopharm vaccine could arrive by the end of the second quarter, and the Moderna shot in the third quarter, he said.
Indonesia aims to inoculate 181.5 million people within a year in an effort to reach herd immunity in a vaccination drive that began in January.
The Indonesian Chamber of Commerce and Industry had pushed the government to authorise the private inoculation drive.
More than 11,500 companies have signed up for the plan, which would see some 7.4 million people vaccinated, Mr Rosan Roeslani, head of the business group, told the same hearing on Monday.
The programme would allow employees of participating companies, plus their family members, to receive free vaccinations at privately-run health centres with shots distributed by Bio Farma.
Indonesia's food and drug agency is currently reviewing the Sinopharm vaccine for emergency use approval, its chief, Dr Penny Lukito, told the hearing. The agency has made no mention of the approval status of the Moderna vaccine.