Asian Insider
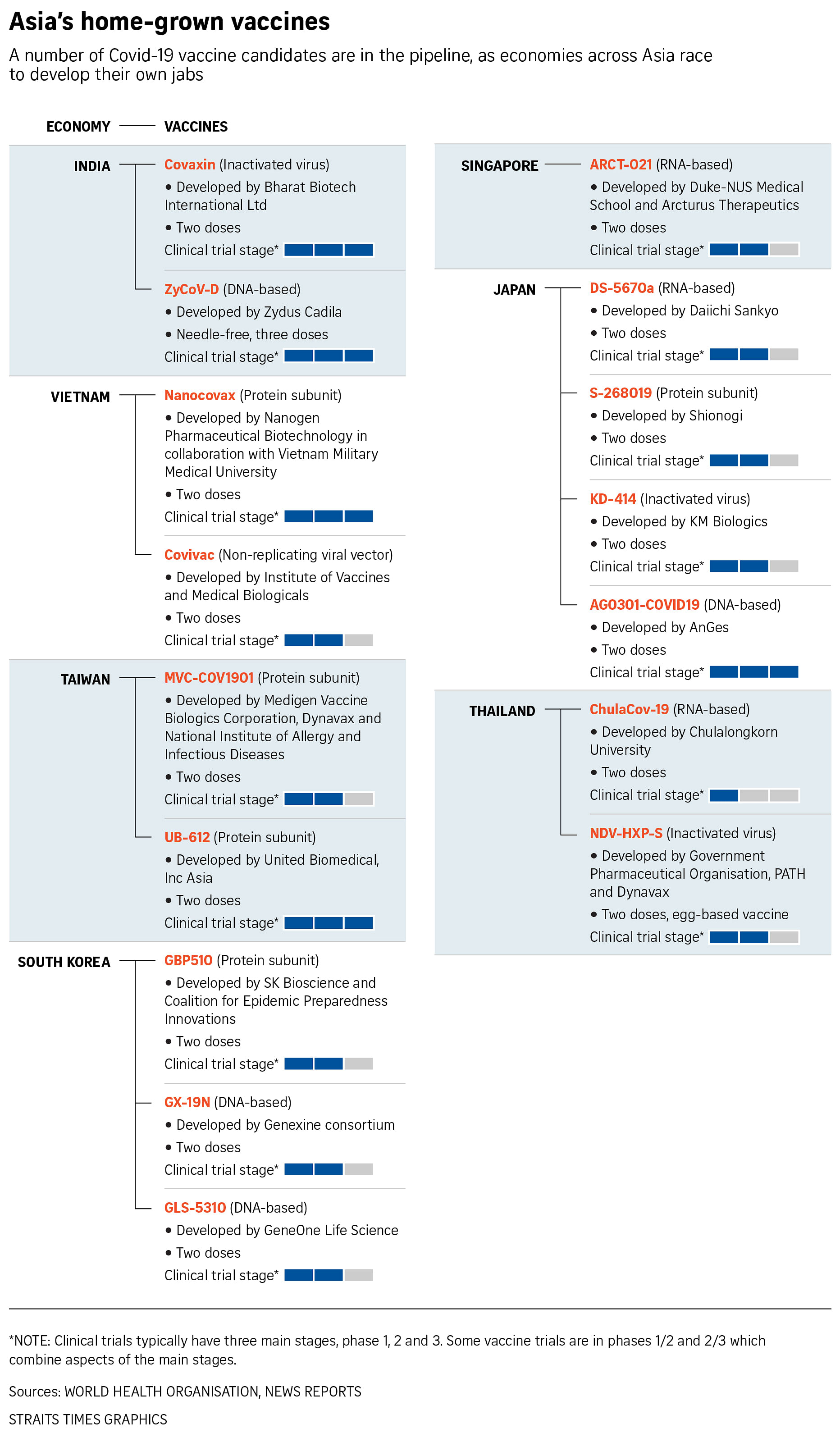
Four in running for Covid-19 vaccines in Japan, but regulations causing lags
Sign up now: Get ST's newsletters delivered to your inbox

A Covid-19 vaccination centre at the Noevir Stadium Kobe in Japan. Japan lags in vaccine development due to its own chequered history.
PHOTO: REUTERS
Follow topic:
TOKYO - Four drugmakers are conducting human clinical trials for Covid-19 vaccines in Japan, though approvals remain months away.
Despite its global reputation as a leader in pharmaceutical drugs, Japan lags in vaccine development due to its own chequered history.
A 1992 court decision that said the government was liable to pay damages to those who suffer adverse side effects led Tokyo to tighten regulatory approval to the point where it became untenable for firms to invest in research.
As a result, Japan has had to play catch-up.
With one in two Japanese having received at least one dose of either the Pfizer or Moderna vaccine, its home-grown vaccines will likely be used for Japan's vaccine diplomacy or as booster immunisation shots to foreign-made vaccines.
Clinical trials are now focused on proving that the vaccines are no less effective than Pfizer or Moderna vaccines, with test subjects to be scouted abroad given the shrinking domestic pool.
Biotech start-up AnGes was the first in Japan to begin human clinical trials in June last year, and has tested its DNA-based vaccine on 500 people.
It was followed by pharmaceutical firm Shionogi, whose recombinant protein vaccine uses genetically modified insect cells to produce the Covid-19 spike protein.
The firm, which is also developing once-a-day pill treatments for Covid-19 patients, said it can produce 120 million doses of vaccine a year after it wins approval.
Daiichi Sankyo aims to roll out its messenger RNA vaccine next year.
KM Biologics, a subsidiary of food giant Meiji Holdings, plans to roll out 35 million doses of its inactivated virus vaccine within six months of reaching approval.