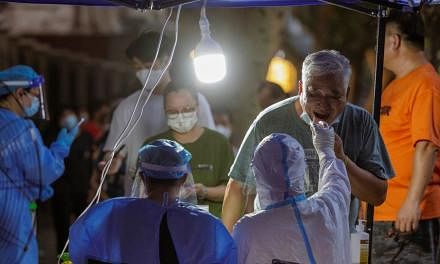
WASHINGTON (BLOOMBERG) - President Donald Trump blamed his predecessor for a nationwide shortage of test kits for coronavirus and claimed to have ended regulations put in place by Barack Obama's administration that limited the development of the diagnostic tools.
"The Obama administration made a decision on testing that turned out to be very detrimental to what we're doing and we undid that decision a few days ago so that the testing can take place in a much more accurate and rapid fashion," Trump said at a meeting on Wednesday (March 4) at the White House to discuss the coronavirus outbreak with airline executives.
He added: "That was a decision we disagreed with - I don't think we would have made it - but for some reason it was made but we've undone that decision."
The Trump administration has come under criticism for the test-kit shortage, which local public health officials have said hampers their ability to survey the US population for the virus. San Francisco's Mayor London Breed called the shortage of kits "a national disgrace" in a letter to Vice-President Mike Pence on Tuesday.
While Trump tried to pin blame on Obama, the initial responsibility for the shortage appears to lie with the Centres for Disease Control and Prevention. Rather than adopt a test used overseas and recommended by the World Health Organisation, the CDC chose to develop and distribute its own, according to reporting by ProPublica. That test didn't work, forcing a scramble by the Trump administration for alternatives.
On Saturday, the FDA issued a policy allowing independent labs to begin using coronavirus tests they develop even before the health agency signs off on what's called an Emergency Use Authorisation. Shortly before Trump was inaugurated, the Obama administration issued guidance on Emergency Use Authorisations, which date to a 2004 law passed after the US anthrax attacks.
Asked to explain his remarks, Trump referred the question to Pence and Centres for Disease Control and Prevention Director Robert Redfield.
Pence said that because of the FDA policy issued Saturday - which he and Redfield described as a Trump order - states can now conduct coronavirus testing in state and university laboratories.
Redfield said that private laboratories used to be able to develop clinical tests and apply them, but "in the previous administration that became regulated. For someone to do that they had to file with the FDA." Thanks to the new FDA policy, he said, "university labs and others can be fully engaged in developing diagnostics." "It was something we had to do and we did it very quickly," Trump said. He said "many, many more sites" and "many, many more people" will be able to be tested.

"You couldn't have that under the Obama rule and we ended that rule very quickly," he said.
Experts on lab testing said they were unaware of any Obama-era rule that would have hindered the administration from authorising lab-developed tests for the coronavirus in an emergency. The FDA has had the authority to authorise tests for emergency use under the 2004 law, signed by President George W. Bush, aimed at advancing medical countermeasures for biological weapons.
"We cannot find a basis for this," said Peter Kyriacopoulos, chief policy officer for the Association of Public Health Laboratories, which represents state and local laboratories. "We are not familiar with the rule they are referring to."
About a decade ago, the FDA grew concerned about what it called "high-risk" lab-developed tests - products that made exaggerated claims unsupported by evidence or relied on falsified data. In 2010, the agency said it would begin reviewing its policy of "enforcement discretion" for the tests.
But the review ended in January 2017, days before Trump was inaugurated, without the FDA issuing any new regulations. The agency published only a "discussion paper" that it said wasn't enforceable, in addition to the guidance on emergency authorisations.