NEW YORK • A few months ago, after a fertility procedure at a Mexican clinic, a healthy baby boy was born in New York to a couple from Jordan. It was the first live birth of a child that has been called - to the dismay of scientists who say the term is grossly misleading - a three-parent baby.
The method used to help the couple is one that reproductive scientists have been itching to try, but it is enormously controversial because it uses genetic material from a donor, in addition to that of the couple trying to conceive. The purpose is to overcome flaws in a parent's mitochondria that can cause grave illnesses in babies.
Mitochondria, the cell's energy factories, are separate from the DNA that determines a child's inherited traits. But mutations in these little organelles can be devastating, resulting in fatal diseases involving the nerves, muscles, brain, heart, liver, skeletal muscles, kidney and the endocrine and respiratory systems that often kill babies in the first few years of life.
The technique that led to the healthy birth was to move the DNA from an egg of the mother, who had mutated mitochondria, and place it in the egg of a healthy donor - after first removing the healthy donor's nuclear DNA from her egg cell. Then that egg, with its healthy mitochondria and the mother's DNA, could be fertilised.
More than a decade ago - before controversy forced the work to stop - researchers had tried a simpler technique. They injected some healthy mitochondria into an egg in an attempt to help with repeated failures at in-vitro fertilisation. It was not a method that could be used to prevent the birth of children with mitochondrial diseases.

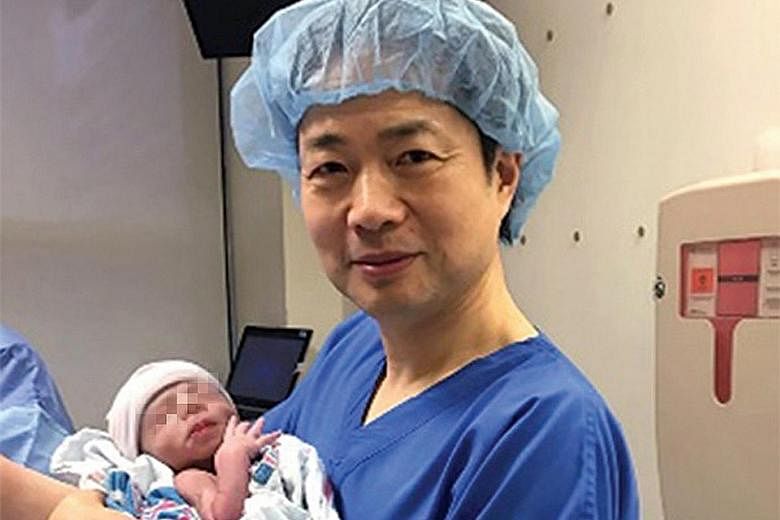
The story of the Jordanian couple's procedure began in 2011 when they came to see Dr James Grifo, a professor of obstetrics and gynaecology at New York University who pioneered the method in studies with mice. He referred them to his former student, Dr John Zhang, medical director of the New Hope Fertility Centre in New York.
Dr Zhang had tried the method in 2003 in China, but the 30-year-old woman's twins were born prematurely and died, though their mitochondria were normal.
When Dr Zhang told the Jordanian couple about the technique, they hesitated. Although they already had a child who was terribly ill with Leigh syndrome, a mitochondrial disease, there was still a chance they could have a normal baby on their own.
A quarter of the woman's mitochondria were mutated, but mitochondria are distributed at random in eggs. If an egg with mostly good mitochondria happened to be fertilised, the baby would be fine. They decided to take their chances.
The couple returned to Jordan and had a baby. But the baby had the same mitochondrial disease, Leigh syndrome.
It is a terrible disease, Dr Zhang said. Babies progressively lose their ability to move and breathe. The baby had a tracheotomy and a feeding tube, he said, and the parents had to suction the baby's lungs every hour.
-
Facts and friction
-
WHAT ARE THE PUZZLE PIECES IN PLAY?
Genetic material of every living being is encoded on the strands of deoxyribonucleic acid (DNA) in the nucleus of the cell.
The nucleus, like the other organelles, floats in the jelly-like cytoplasm inside the cell.
In addition to the nucleus, human cells also contain mitochondria, which provide the cell with energy.
The endosymbiotic theory suggests that mitochondria used to be single-celled organisms that were incorporated into human cells, in the early age of cellular life, billions of years ago. Therefore, mitochondria also contain DNA, known as mitochondrial DNA (mtDNA).
mtDNA is inherited almost exclusively from the female parent.
Unlike nuclear DNA, mtDNA does not control the appearance or genetic make-up of a person.
OTHER SIMILAR METHODS
Pronuclear transfer was given the green light in the United Kingdom last year.
This technique involves fertilising both the egg of a mother with faulty mitochondria, and a donor egg with healthy mitochondria.
The nuclear material of both eggs is extracted at an early stage, before the fertilised eggs develop into embryos.
The chromosomes containing the parents' DNA are then implanted into the donor egg.
A previous three-parent technique used cytoplasmic transfer to treat infertility.
The cytoplasm from a donor would be introduced into the mother's egg, with some mitochondria - and mtDNA - coming along for the ride.
Preliminary studies with mice suggest that it could be possible eventually to develop embryos using the genetic material from two eggs or two sperm, which would be a boon for same-sex couples who want genetically related children.
OBJECTIONS
Some religious people have qualms about assisted reproductive techniques because they believe that a fertilised egg constitutes a distinct human life, and consider the discarding or destruction of embryos in the process to be unacceptable.
Some also believe that conception should take place only during natural sexual relations between a married couple.
Earlier attempts at using three-parent techniques to treat infertility or genetic disorders in the 1990s were called off for medical reasons, when miscarriages or developmental disabilities arose.
The scientific community was also concerned that the unusual genetic code of a female child with three parents' DNA could be passed down to her own children and subsequent generations.
LEGALITY
Three-parent techniques have been off the table in the United States since 2002, due to safety and ethical concerns by the Food and Drug Administration.
In 2015, Britain became the first country to legalise pronuclear transfer.
Annabeth Leow
The first child died at age six; the second child at eight months.
The couple returned to Dr Zhang, ready to try the mitochondrial transfer technique. New Hope Fertility Centre has a clinic in Mexico, so he suggested doing the procedure there because it is effectively banned in the United States.
Six months into the pregnancy and the woman said she knew this baby was different. It kicked constantly - the others, affected even in the womb, had hardly moved.
Now the boy is five months old, healthy and has normal mitochondria. The birth was first reported on Tuesday by New Scientist magazine.
Specialists in reproductive medicine say they hope this success will change attitudes towards mitochondrial transfers.
NYTIMES