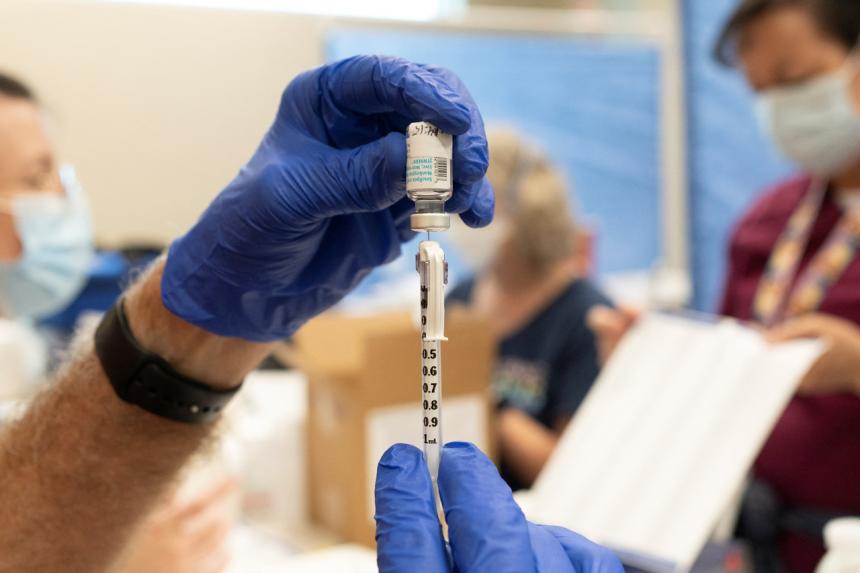
NEW YORK - Nearly four months after the first report of monkeypox in the United States, the virus is showing promising signs of retreat, easing fears that it may spill over into populations of older adults, pregnant women and young children.
Supplies of the vaccine have improved, and federal health officials have begun clinical trials to gain a better understanding of who benefits, and how much, from both the vaccine and the drug used to treat those who become infected.
That's the good news. But unhappily, case numbers are accelerating in a few states and jurisdictions, including Indiana, Virginia and Massachusetts. Black and Hispanic men make up nearly two-thirds of the infected, but only about one-fourth of those vaccinated so far.
"Our progress is incredibly uneven," said David Harvey, the executive director of the National Coalition of STD Directors.
"This outbreak is far from finished," he added.
Recent reports suggest that a single dose of the vaccine, Jynneos, may not be protective enough, raising fresh concerns about the Biden administration's plan to distribute fractional doses.
And federal health officials have warned that the virus could become resistant to tecovirimat, the only safe treatment for those who are infected.
"When you only have one drug in your armamentarium, that can be somewhat precarious," said Dr Anthony Fauci, the Biden administration's top medical adviser. "But you've got to go with what you have at the same time as you try and develop additional drugs."
As of Friday, there were nearly 25,000 cases of monkeypox in all 50 states, the District of Columbia and Puerto Rico. The United States accounts for nearly 40 per cent of the global tally.
But new cases have been decreasing steadily for weeks, to a daily average of 208 on Sept 22 from more than 500 in early August.
The Los Angeles Department of Public Health recently confirmed the nation's first death from monkeypox, in a severely immunocompromised individual. Health officials in Texas are investigating another death that may be related to the infection.
Two cases of encephalomyelitis - inflammation in the brain and spinal cord - have been reported, both in previously healthy gay men in their 30s.
Overall, however, federal health officials are optimistic that the epidemic is waning. While testing and vaccines will continue to be important, officials envision a future in which monkeypox is not gone, but manageable with contact tracing, vaccination and early treatment.
"I think it's going to look a little bit more like more episodic cases, smaller clusters," said Dr Demetre Daskalakis, the deputy coordinator of the White House's monkeypox response.
The recent decline is most likely the result of a combination of vaccinations, immunity gained from infection in the population most at risk, and a change in behaviour in this group, Dr Daskalakis said.

In a survey conducted by the Centres for Disease Control and Prevention in August, roughly half of men who have sex with men said they had reduced the number of their partners and one-time sexual encounters.
But falling case numbers may soon lead these men to believe that the threat has passed.
"We can't ask people to change their behaviour forever," Dr Daskalakis said. "That didn't really work with HIV, so it's not going to work here, either." Vaccination is likely to be a more effective containment strategy in the long term, he added.
As of Sept 20, health officials had administered nearly 700,000 doses of Jynneos in the 48 jurisdictions for which data were available. While that is a substantial improvement over the early weeks of the outbreak, it accounts for only 22 per cent of the doses needed to protect the 1.6 million Americans estimated to be at high risk.
Even as infections decline, the proportion of cases among Black and Hispanic men has grown to 70 per cent in mid-September from 37 per cent in late May. Yet Black men have received less than 9 per cent of the doses administered so far, and Hispanic men about 16 per cent.
Federal health officials are intensifying efforts to reach high-risk groups and have vaccinated at least 11,000 attendees at large gatherings where Black and Hispanic men congregate, such as Atlanta Black Pride.
The CDC has announced a new program that would make up to 10,000 vials of vaccine - or 50,000 doses, under the new dose-sparing strategy - available to communities where hesitancy, language barriers, immigration status or other obstacles prevent widespread vaccination.
Eligibility for the vaccine is scattershot by location, and the criteria often opaque, according to an analysis by the Kaiser Family Foundation. Some states, such as Indiana and New Mexico, offer no information online about who qualifies. Laboratory and health care workers who may be exposed to the virus are eligible in only 18 states and cities.
Many men at high risk have opted for a single dose, which may not be sufficiently protective. Although the proportion of second doses has increased, so far 77 per cent of administered doses are first doses.

A new study suggests that will not be enough to prevent infection or severe symptoms. Dutch researchers found that one full dose of Jynneos produces low levels of antibodies to monkeypox.
Two full doses are better but still "modest," said Dr Marion Koopmans, the head of virology at Erasmus Medical Centre in Rotterdam, the Netherlands, who led the study.
"It does raise the question how good protection will be," she said. "Since we don't know a whole lot about this, I do think we really need to figure out what's going on."
In a bid to stretch the vaccine supply, the Biden administration has embraced a dose-splitting strategy, in which one-fifth of a regular dose is delivered into the skin - a so-called intradermal method - rather than the fat underneath. This approach has been tried in other instances of vaccine shortage.
But activists and some scientists have decried the administration's reliance on fractional dosing, noting that federal officials moved slowly to make available millions of Jynneos doses held by the manufacturer in Denmark.
"What's so bizarre about this whole thing is we should have never gotten into the situation," said Mr James Krellenstein, a founder of PrEP4All, a group that promotes access to HIV care.
There is minimal research to support fractional doses instead of the full regular doses, he noted: "They may be equivalent, but there's a real good chance that they're not."
The Dutch team did not look at how well a one-fifth dose of Jynneos protects against monkeypox. But in an earlier study, they tested a bird flu vaccine similar to Jynneos and found that two fractional doses produced much lower levels of antibodies than two full doses.
Still, it's possible that a combination of one full dose and one fractional dose may work well, Dr Koopmans said.
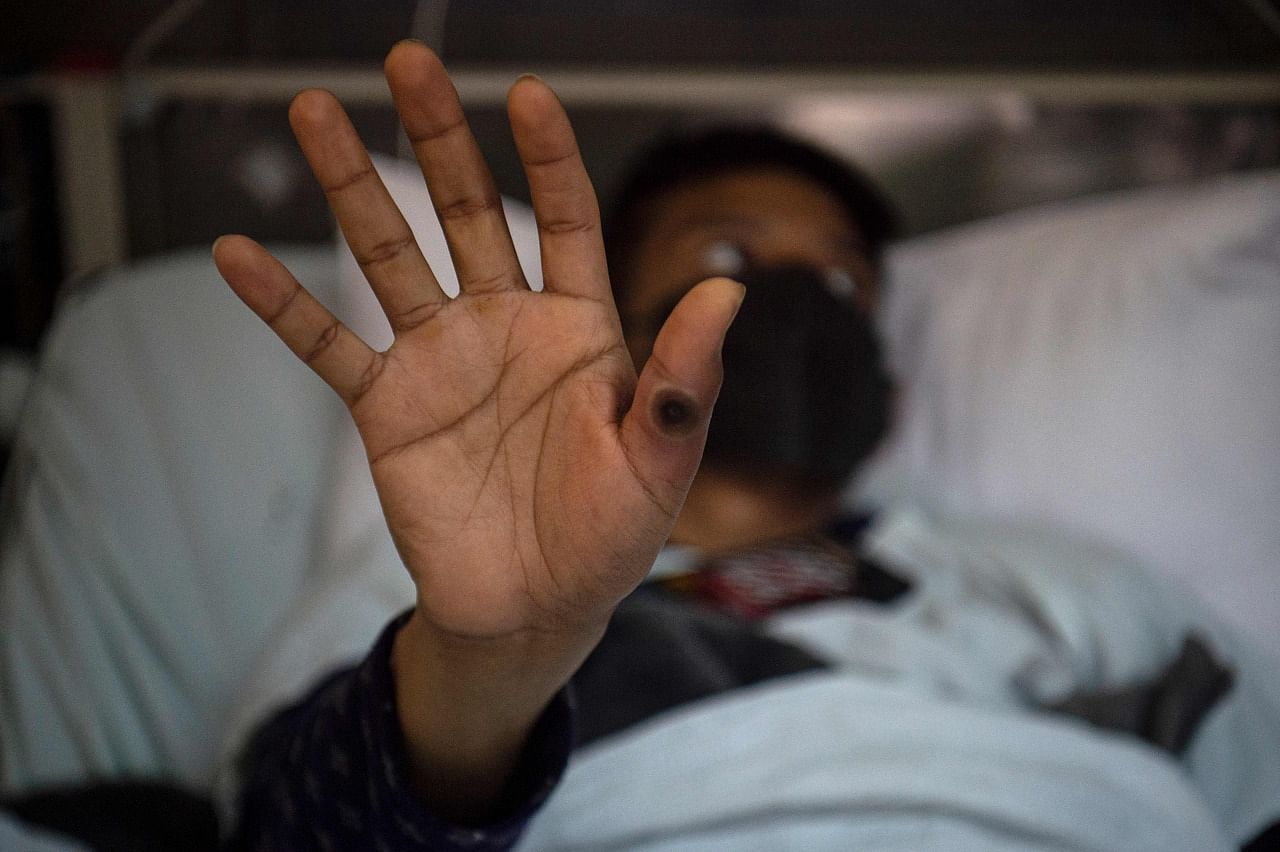
Little is known about the effectiveness of regular doses, let alone fractional doses, because Jynneos was approved mainly on the basis of animal data. But the evidence so far suggests that two doses are better than one, said Dr Peter Marks, the Food and Drug Administration's top vaccine regulator.
"Having two doses of Jynneos was the correct way to go here, and the fact that the intradermal route allowed us to have a sufficient number of doses to move forward in that direction, I think, was a smart idea," he said.
"We're working in a public health emergency," he added. "I think we're doing our best with the data that we have in hand, and the data that we trust, and the data as it emerges."
There is some evidence that a third shot given a year after the first two doses provokes a vigorous immune response. If that turns out to be true, a three-dose regimen may be ideal to manage monkeypox infections in the long term. Dr Marks said federal scientists are still debating whether to test third doses.
A new trial led by the National Institutes of Health, which began earlier this month, will enrol 200 adults and compare the standard dose with intradermal delivery of one-fifth and one-tenth doses.
If the fractional doses prove to be comparably effective, the dose-splitting approach would greatly expand world supply, including in countries where the vaccine is currently unavailable.
Researchers will collect information on antibody levels in the immunised participants. But they will not be tracking other immune cells that may be equally important for protection from the virus, according to Dr John Beigel, the NIH researcher leading the trial.
"This was a decision for expediency," he said.
A separate NIH trial will test how well tecovirimat, also called Tpoxx, works in 500 adults and children infected with monkeypox.
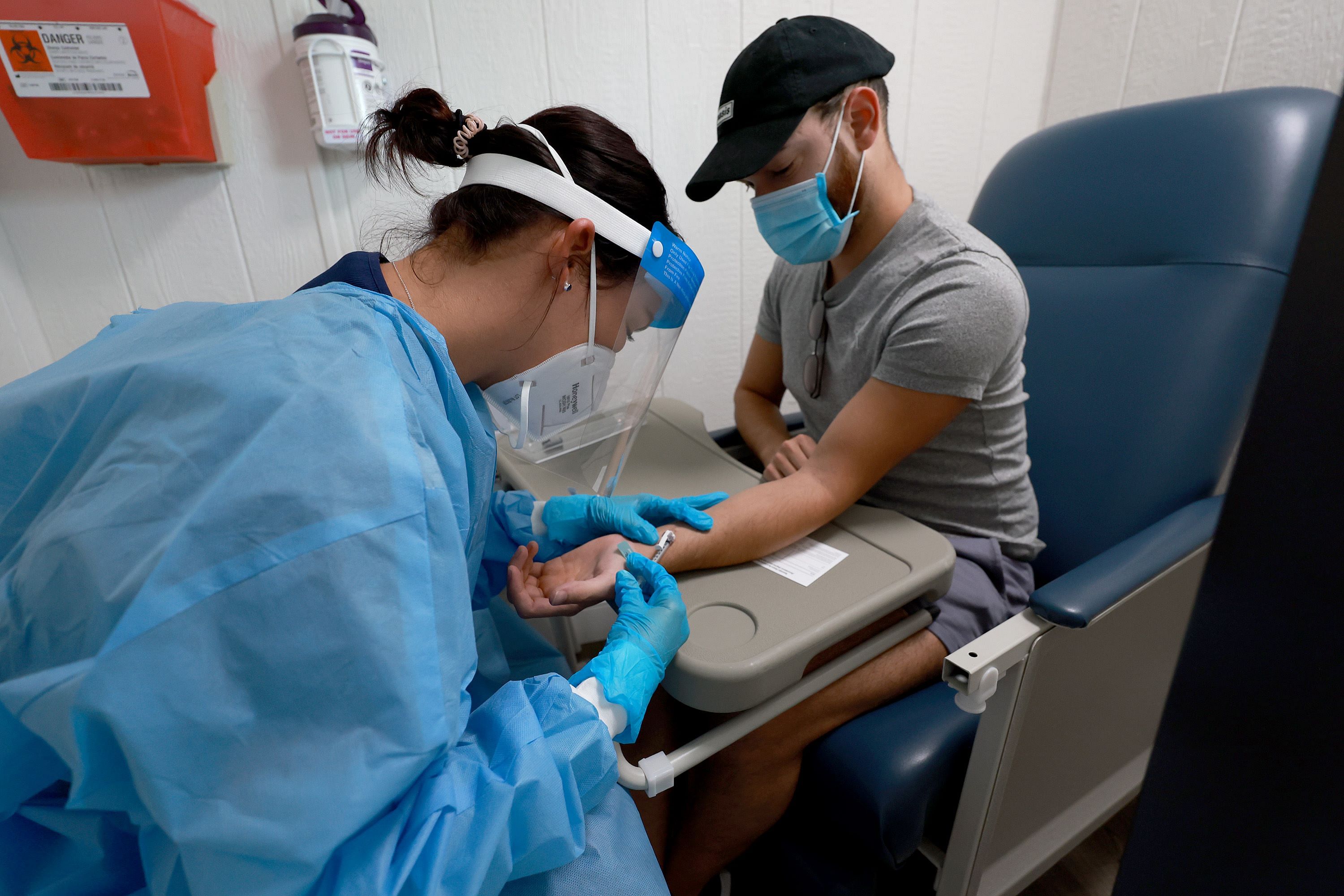
Tecovirimat is the only drug used to treat monkeypox in the United States, as the alternatives can have toxic side effects. The drug was approved in 2018 on the basis of animal studies, and has never been tested rigorously in people.
Small clinical studies, as well as recent anecdotal observations of patients, suggest that the drug works well. A small percentage of patients experience minor side effects, such as headache and nausea.
Given the early data, the Biden administration has been sharply criticised for making it too difficult for clinicians to prescribe the drug. And the CDC has urged clinicians to reserve tecovirimat for patients who are severely immunocompromised, pregnant or breastfeeding, or who have lesions in certain sensitive areas, as well as for children younger than 8.
The decision to limit access is rooted in the fear that indiscriminate use could lead to Tpoxx-resistant monkeypox, federal officials said. Several studies suggest that even small genetic changes could leave the virus resistant, according to the FDA.
The new trial should offer a clearer picture of the risk. "We want to make it much easier, and with much more confidence, to make Tpoxx available for people who are infected," Dr Fauci said. NYTIMES