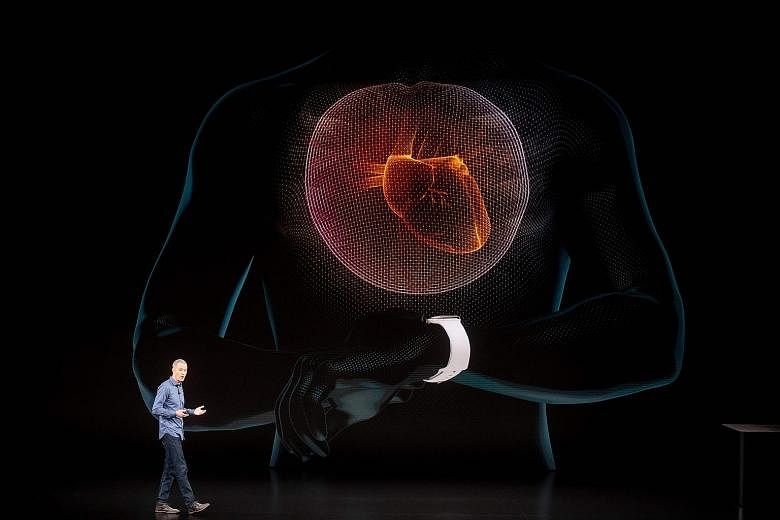
The newest Apple Watch can now flag potential problems with your heartbeat - a feature that has been cleared by the United States Food and Drug Administration (FDA) and that Apple is marking as a major achievement.
But some doctors say including heart-monitoring tools in such a popular consumer product could prompt unnecessary anxiety and medical visits.
Already a subscriber? Log in
Read the full story and more at $9.90/month
Get exclusive reports and insights with more than 500 subscriber-only articles every month
ST One Digital
$9.90/month
No contract
ST app access on 1 mobile device
Unlock these benefits
All subscriber-only content on ST app and straitstimes.com
Easy access any time via ST app on 1 mobile device
E-paper with 2-week archive so you won't miss out on content that matters to you