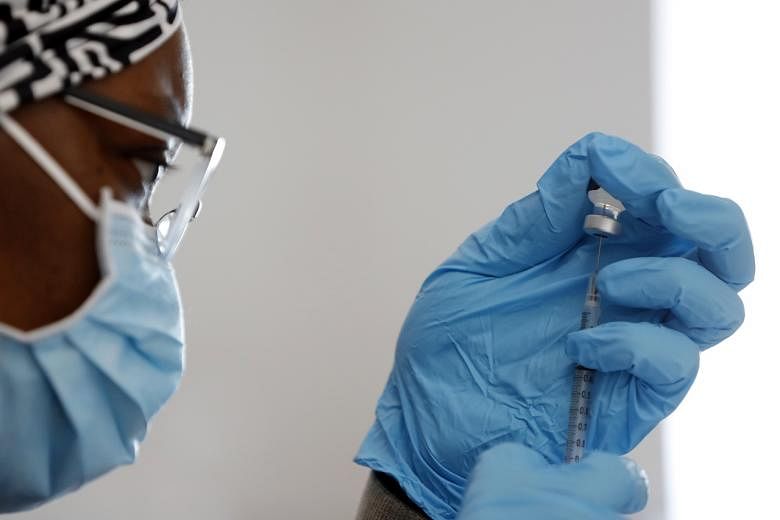
(NYTIMES) More vaccines are coming soon.
The one-dose coronavirus vaccine developed by Johnson & Johnson was reviewed on Friday by an advisory committee for the US Food and Drug Administration (FDA), and authorised over the weekend. The FDA approved the emergency use of the vaccine, which has been shown to strongly protect recipients against severe disease and death from the coronavirus.
Already a subscriber? Log in
Read the full story and more at $9.90/month
Get exclusive reports and insights with more than 500 subscriber-only articles every month
ST One Digital
$9.90/month
No contract
ST app access on 1 mobile device
Unlock these benefits
All subscriber-only content on ST app and straitstimes.com
Easy access any time via ST app on 1 mobile device
E-paper with 2-week archive so you won't miss out on content that matters to you