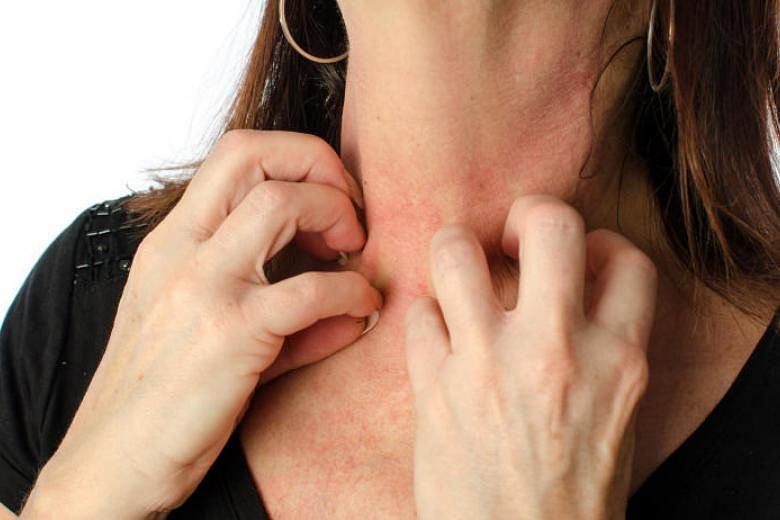
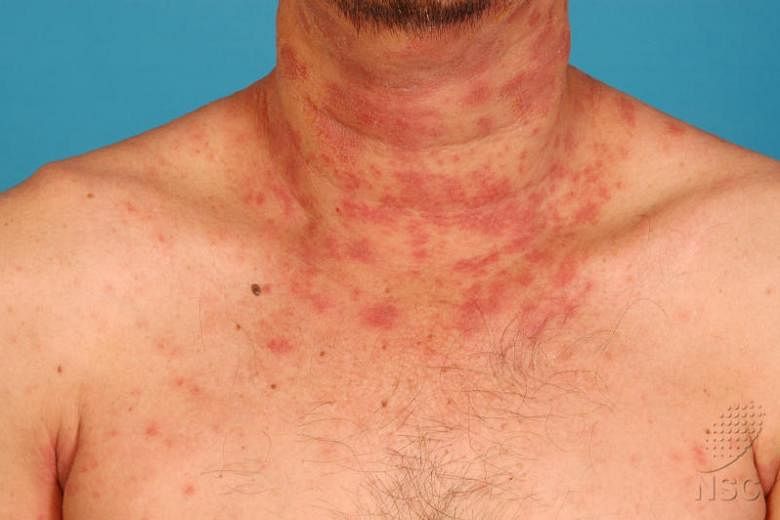
SINGAPORE - A newly approved first-of-its-kind biologic drug may bring hope to patients who are having a hard time bringing their moderate to severe atopic eczema under control.
Some patients at both public and private institutions, including adolescents, have been treated with the newly available Dupilumab, sold under the brand name Dupixent, which has been shown to be relatively safe and effective in international trials that included Singapore. The Health Sciences Authority approved its use in adult patients about two months ago.
Already a subscriber? Log in
Read the full story and more at $9.90/month
Get exclusive reports and insights with more than 500 subscriber-only articles every month
ST One Digital
$9.90/month
No contract
ST app access on 1 mobile device
Unlock these benefits
All subscriber-only content on ST app and straitstimes.com
Easy access any time via ST app on 1 mobile device
E-paper with 2-week archive so you won't miss out on content that matters to you