SINGAPORE (THE BUSINESS TIMES) - The Singapore Exchange (SGX) on Wednesday morning (April 22) flagged unusual price movements in the shares of cancer diagnostics firm Biolidics.
The Catalist-listed counter had surged as much as 54.6 per cent or 29.2 cents to hit 82.7 cents at around 10am.
It then eased back to trade at 72.5 cents - an increase of 35.5 per cent or 19 cents - by the midday break. About 79.7 million shares had changed hands by then, making Biolidics one of the most heavily traded by volume for the morning session.
Biolidics, which now has a market cap of about $139.3 million, requested a trading halt at 1.09pm, pending the release of an announcement.
Meanwhile, its substantial shareholder Clearbridge Health also saw its share price surge in the morning. The integrated healthcare group's shares jumped 69.6 per cent or 11 cents to 26.8 cents at 10am. The counter was trading at 24.5 cents as at the midday break, up 55 per cent or 8.7 cents, after 109 million shares changed hands.
Clearbridge Health's wholly-owned subsidiary Clearbridge BSA has a 24.8 per cent stake in Biolidics as at March 18, according to Biolidics' annual report.
Asked if there was a development at Clearbridge that might explain the increase in its share price, group chief executive officer Jeremy Yee said no, but noted that Clearbridge is a shareholder of Biolidics.
On Wednesday, SGX asked Biolidics whether it was aware of any information not previously announced concerning the company, its subsidiaries or associated companies which might explain the trading.
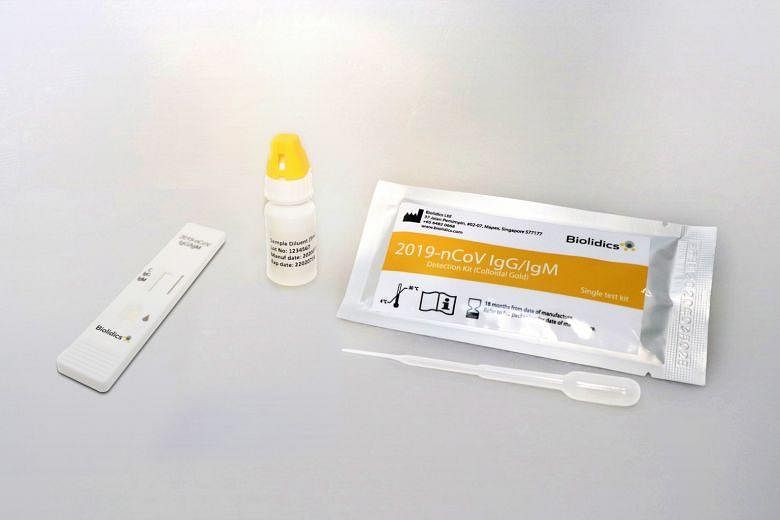
Before the bourse operator's query, the Singapore-based company's latest regulatory filing was made on Monday, when it said it can now distribute, market and sell its Covid-19 rapid test kits in the US.
Biolidics also separately announced in recent weeks that it had received official approval for the novel coronavirus test kit in the European Union, in the Philippines and in Singapore.
Earlier this month, SGX asked the company why it had disclosed the approval from Singapore's Health Sciences Authority (HSA) only on March 30 - at least a week after HSA already announced it - especially in light of the seriousness of the Covid-19 situation and a worldwide shortage of Covid-19 test kits.
In response, Biolidics said it only became material to disclose the information after it was certain the kits could be commercialised.