With not one, not two, but three Covid-19 vaccines applying for emergency use authorisation across the globe, and several others in clinical trials, including a home-grown candidate, Singapore can be sure it will not have to put all its eggs in one basket to keep its people safe from the coronavirus. And it may also not have too long to wait.
Professor Ooi Eng Eong, deputy director of Duke-NUS Medical School's Emerging Infectious Diseases Programme, said: "We should use whatever vaccines with well-proven safety and efficacy as soon as possible to protect our population and return to our normal lives as much as, and as soon as possible."
In the past few weeks, news of promising interim late-trial results for three vaccine candidates has come thick and fast, helping to inject some joy into a world weighed down by a pandemic that has infected more than 61 million people and claimed over 1.4 million lives.
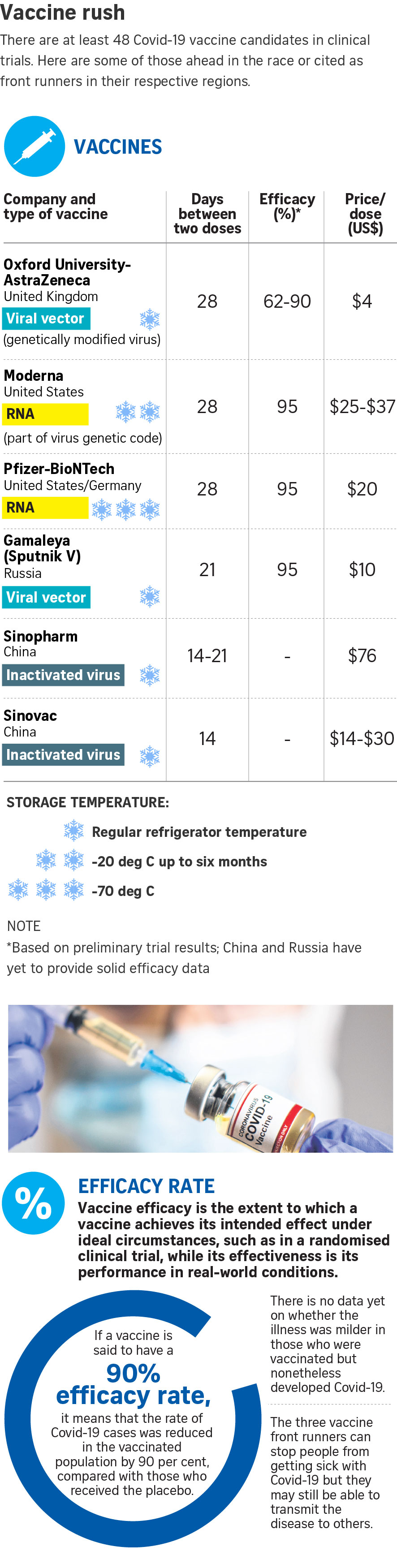
Pharmaceutical giant Pfizer and its German partner, BioNTech, were first off the blocks, announcing on Nov 9 that their vaccine candidate was more than 90 per cent effective in preventing people from getting the disease. This went up to 95 per cent when it released the first set of complete results on Nov 18.
Fast on its heels was US pharmaceutical firm Moderna, which said on Nov 16 that its vaccine candidate had demonstrated an efficacy rate of 94.5 per cent.
AstraZeneca was third in the vaccine race to announce it had a potential winner on its hands.
Last Monday, it said that the vaccine candidate it is developing with Oxford University had an efficacy rate of up to 90 per cent if a lower first dose was used with a regular second dose. Otherwise, with two standard doses, the efficacy rate was 62 per cent.
"To achieve 90 per cent to 95 per cent vaccine efficacy in outbreak conditions with a novel pathogen within a year of discovery is as remarkable as two golfers playing on a completely unfamiliar green, who have just hit holes-in-one," said Associate Professor Lim Poh Lian, the head of the Travellers' Health and Vaccination Clinic at Tan Tock Seng Hospital.
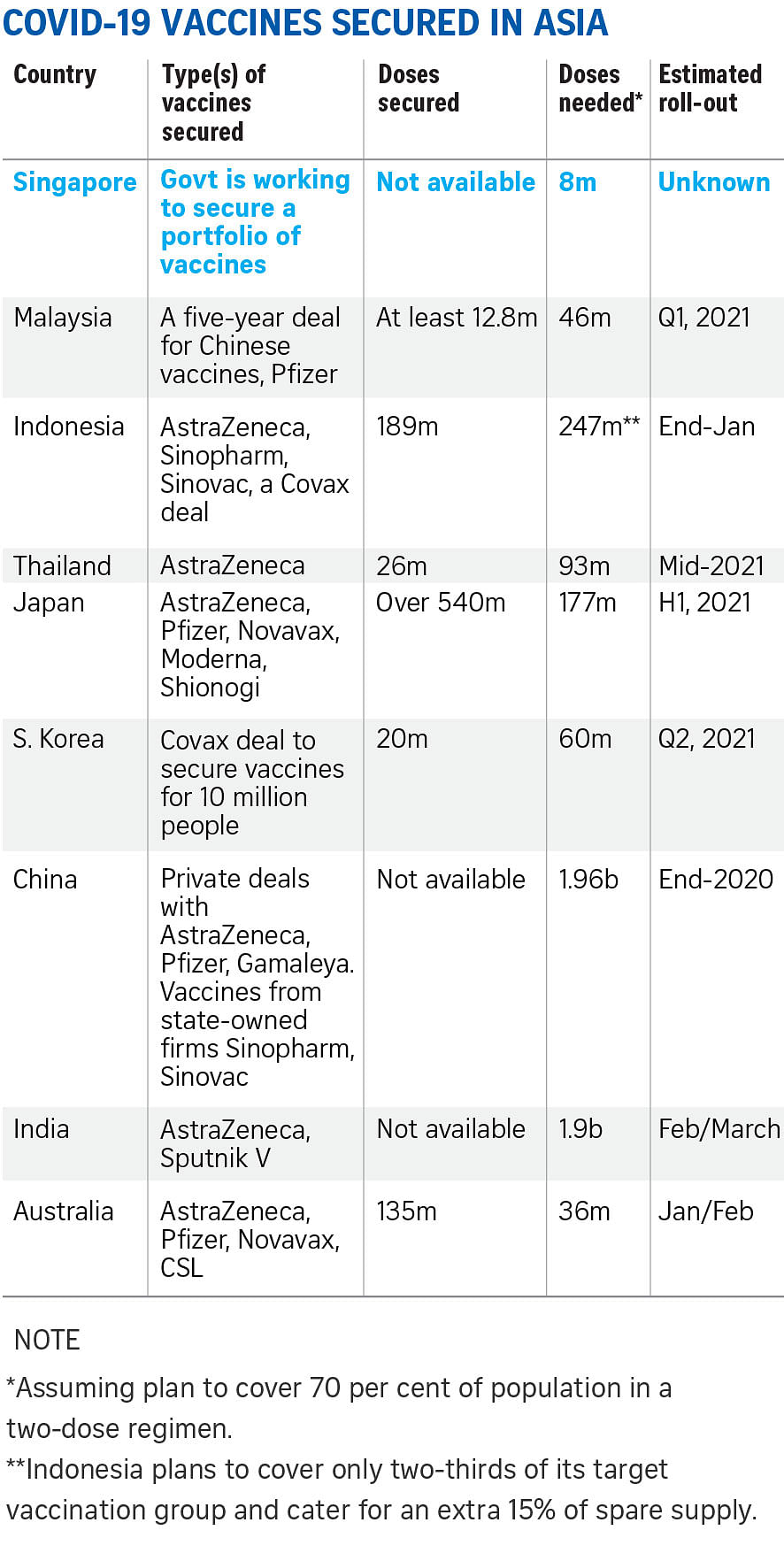
Health Minister Gan Kim Yong has said Singapore will work towards "securing a portfolio" of Covid-19 vaccines to cater to different segments of the population.
Asked which vaccine he was most excited about, Prof Ooi, principal investigator for Lunar- CoV19, the vaccine candidate being developed by Arcturus Therapeutics in the United States and the Duke-NUS Medical School here, replied: "I will take the first available licensed vaccine."
News that the three vaccine candidates can be 90 per cent to 95 per cent effective gave cause for optimism that the world could turn the corner in the virus battle. However, while the vaccines do prevent most people from falling ill with Covid-19, there is no data to show they stop a person from being infected, then passing the infection on even without getting sick; or how long they last.
"Asymptomatic Sars-CoV-2 infection in vaccinated individuals could still allow the virus to spread in our community and cause Covid-19 in unvaccinated individuals," said Prof Ooi.
Experts have stressed that no vaccine is perfect. So, while a good vaccine boosts a person's immune system to give them a headstart in fighting a disease, it may not confer 100 per cent protection, nor will it work on everyone. It can also wear off in time. People who received a shot against mumps or whooping cough, for instance, may still pass it on.
Even so, when enough people are vaccinated, and the virus cannot travel as easily from person to person - the entire community is less likely to get the disease. Prof Ooi said it would take time to determine if any vaccine can prevent the Sars-CoV-2 infection.
"We should thus sustain a pipeline of new vaccines so that we can find those that are most effective at sustaining protection against Covid-19 and possibly even eliminating Sars-CoV-2 from the human population," he said.
At a webinar hosted by The Straits Times on Wednesday, Singapore's chief health scientist, Professor Tan Chorh Chuan, said that Covid-19 vaccines on the cusp of being approved would completely shift the battle against the pandemic but they cannot eliminate all other measures.
"It'd be a game changer because it'd allow us to blunt, very substantially, many of the negative impacts of the Covid-19 pandemic," he said.
"But while it will be a game changer, it will not be a silver bullet. As all of us know, the supply will be limited."
It will probably be a year or two before enough doses are made available to vaccinate enough people around the world, he said.
In the meantime, the public will still have to adhere to safe distancing measures as there will still be a "very substantial risk of transmission and outbreaks".
Prof Tan, who is part of a 14-member committee formed recently to prioritise the people who should be given the vaccines as they become available, said a Covid-19 vaccine will allow the world to protect healthcare workers and older, more vulnerable people who are at higher risk of mortality.
One of the problems that health systems in many places have faced is that their medical staff become infected in the course of their work or in the community, thus reducing the healthcare workforce when it is most needed, he said.
Racing ahead
Right now, human trials of at least 48 Covid-19 vaccine candidates are in progress.
The success in harnessing new technology marks a turning point in the field, but also underscores that the hunt for a vaccine has entered uncharted territory.
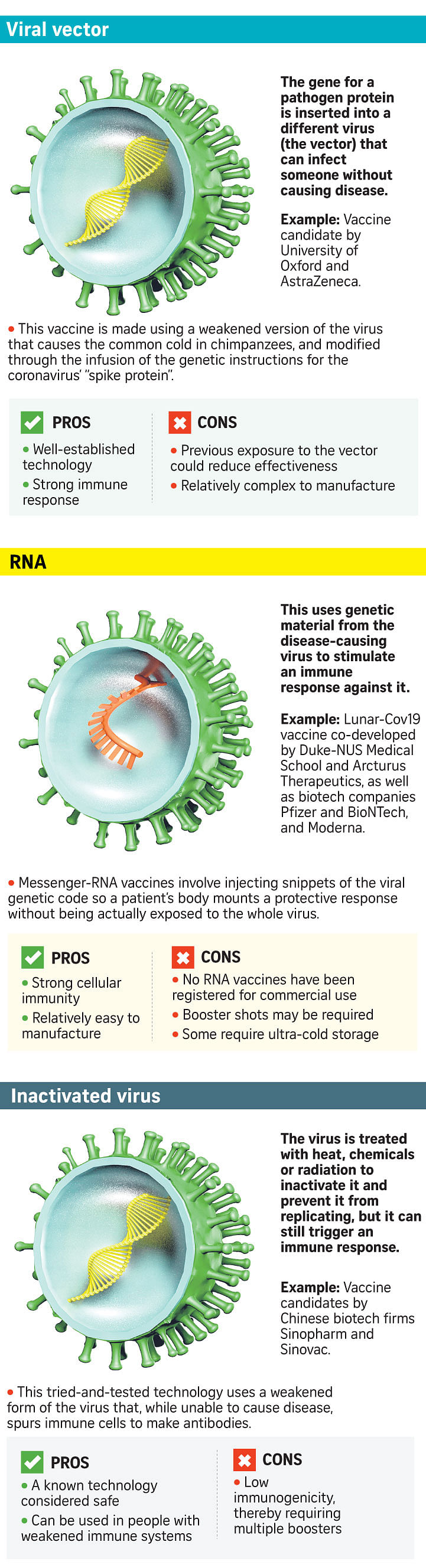
Pfizer and Moderna's vaccines are the most novel, being based on messenger RNA (mRNA), a genetic blueprint which instructs human cells to make the coronavirus' spike protein to induce an immune response.
While DNA and RNA vaccines have been developed against other diseases, such as HIV, and gone through early stage trials, none has been approved for use in humans.
The AstraZeneca-Oxford vaccine is made from a cold virus known as an adenovirus that is derived from chimpanzees - so it is harmless to people, and genetically modified to carry a gene for the spike protein. Such vaccines have yet to be licensed for use in humans, though this technology has been around for many years.
The body makes use of the information encoded in the spike gene to make the spike protein, which then trains the immune system to recognise and react against this characteristic feature of Sars- CoV-2, said Prof Ooi.
The difference is that the vaccine developed by AstraZeneca and Oxford makes use of chimpanzee adenovirus to "carry" the spike gene while the other two vaccine candidates constructed the spike gene in the form of RNA, which is then taken up by the cells as a template for making the spike protein.
The form that the spike gene takes in these vaccines means that their storage conditions differ.
In the mRNA vaccines, the RNA of the spike gene is packaged inside lipid (fat) nanoparticles, which are less stable and, depending on the chemical composition of the lipids, would require lower temperatures to remain stable during shipment and storage.
As for the AstraZeneca/Oxford vaccine, the spike gene is encased inside the protein shell of the adenovirus, which is among the most stable forms of viruses in the world, and can thus be shipped and stored under refrigerated conditions.
This is why experts have said it is likely to have a wider roll-out than, say, Pfizer's mRNA vaccine, which must be stored in ultra-cold conditions.
However, doubts have been raised over the efficacy of this vaccine, as the developers have acknowledged an error in the vaccine dosage that led to some study participants getting a lower first dose, though this regimen turned out to be a winner. These are the people aged 55 and below, so it is not clear if this dosing regime would offer the same coverage for older people.
Storage that requires special freezers presents a major logistical challenge. Cost is also a factor in vaccine distribution.
AstraZeneca has promised to make no profit from its vaccine while the pandemic lasts. Its dose is reportedly cheaper - at about US$4 (S$5.40), compared with around US$20 for Pfizer's vaccine and between US$25 and US$37 for Moderna's vaccine.
The vaccine candidate from Arcturus and Duke-NUS is also based on mRNA. Arcturus has said it could be shipped out in the first quarter of next year.
Are these vaccines safe?
The speed at which Covid-19 vaccines are being developed has been described as incredible and extraordinary.
It is an unprecedented pace made possible by parallel processing, said Prof Ooi.
"Conventionally, each step of the vaccine development is done serially, one after another. This is so that vaccine developers can avoid committing large financial resources prematurely to a vaccine that may not work as desired," he said.
But the urgency of the Covid-19 pandemic caused many companies and teams to take on the financial risks and accelerate vaccine development. "Importantly, there have been no shortcuts in determining vaccine safety and efficacy."
Scientists have also been able to accelerate the Covid-19 vaccine development process because of vast improvements in vaccine technology, said Prof Tan.
The mRNA and adenoviral vector vaccines, for instance, can be designed, developed and tested much faster than by traditional methods.
He pointed out that the more common side-effects, such as pain at the injection site or fever after vaccination, as well as rare side-effects, will be picked up at the trials as they have been done at a very substantial scale. However, "some of the very rare side-effects and some of those side-effects which may take some time before they manifest will not be picked up right now".
This is why the planning does not end at the vaccination stage. "We track the sort of side-effects that arise following vaccination and try to pick up as early as possible any signs of very rare or serious kinds of problems, and that then we take action very quickly," he said.
"This is an area that regulators and medical systems around the world are looking at very carefully and there will be very intensive surveillance, if and when vaccinations begin."
At the start, Covid-19 vaccination could be approved or recommended for only some groups, for which data has shown the vaccine to be safe and effective, he added. Prof Lim, who is also a senior consultant at the National Centre for Infectious Diseases and a member of the Ministry of Health's expert committee on Covid-19 vaccination, said there is much hard work ahead, with regard to scrutinising vaccine safety, tolerability and duration of protection.
The experts will also need to analyse vaccine safety and efficacy in different groups, such as older adults, children, pregnant women, immunocompromised persons and those of various ethnicities, she said.
"There will be even harder work with practical aspects of vaccine delivery, and making it accessible to people who need it, and people who want it," said Prof Lim. "But there is good reason that with these vaccine clinical trial results, we finally have real hope that help is coming."